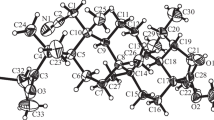
α,β-Unsaturated lupane and 19β,28-epoxy-18α-oleanane aldehydes were used in the synthesis of triterpenoids bearing substituted 1,2-azole moieties (1-acetyl-3-methyl-4,5-dihydro-1H-pyrazole and 3-methyl-4,5-dihydroisoxazole) at the rings А and Е. The route of synthesis for these 1,2-azole derivatives of triterpenes included an aldol condensation of α,β-unsaturated aldehydes with acetone, the products of which (α,β-unsaturated methyl ketone and β-hydroxy ketone) underwent a further cycloaddition reaction with acetylhydrazide and hydroxylamine. Cytotoxic activity studies of the synthesized compounds against seven cancer cell lines (Hep-2, HCT116, MS, RD TE32, A549, MCF-7, and PC-3) showed that the highest cytotoxicity (IC50 0.66–11.97 μM) against all tested cell lines was exihbited by 19β,28-epoxy-18α-oleanane aldehyde and the products of its condensation reactions with acetone and acetylhydrazide.


Similar content being viewed by others
References
Csuk, R. Expert Opin. Ther. Pat. 2014, 24, 913.
Bednarczyk-Cwynar, B.; Günther, A. Curr. Med. Chem. 2017, 24, 2205.
Borkova, L.; Hodon, J.; Urban, M. Asian J. Org. Chem. 2018, 7, 1542.
Sousa, J. L. C.; Freire, C. S. R.; Silvestre, A. J. D.; Silva, A. M. S. Molecules 2019, 24, 355.
Yamansarov, E. Yu.; Saltykova, I. V.; Kovalev, S. V.; Petrov, R. A.; Shkil’, D. O.; Seleznev, E. I.; Beloglazkina, E. K.; Majouga, A. G. Russ. Chem. Bull., Int. Ed. 2019, 68, 855. [Izv. Akad. Nauk, Ser. Khim. 2019, 855.]
Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C. L.; Yli-Kauhaluoma, J. Med. Chem. Commun. 2014, 5, 445.
Kvasnica, M.; Urban, M.; Dickinson, N. J.; Sarek, J. Nat. Prod. Rep. 2015, 32, 1303.
Sidova, V.; Zoufaly, P.; Pokorny, J.; Dzubak, P.; Hajduch, M.; Popa, I.; Urban, M. PLoS ONE 2017, 12, e0171621.
Uzenkova, N. V.; Petrenko, N. I.; Shakirov, M. M.; Shul’ts, E. E.; Tolstikov, G. A. Chem. Nat. Compd. 2005, 41, 692. [Khim. Prirod. Soedin. 2005, 571.]
Zorina, A. D.; Nikiforova, N. S.; Zarubaev, V. V.; Marchenko, S. A.; Selivanov, S. I.; Starova, G. L.; Mehtiev, A. R.; Rodionov, E. I.; Rodionova, A. A.; Trifonov, R. E. Mendeleev Commun. 2019, 29, 500.
Santos, R. C.; Salvador, J. A. R.; Marín, S.; Cascante, M. Bioorg. Med. Chem. 2009, 17, 6241.
Thi, T. A. D.; Tuyet, N. T. K.; The, C. P.; Nguyen, H. T.; Thi, C. B.; Duy, T. D.; D’hooghe, M.; Nguyen, T. V. Bioorg. Med. Chem. Lett. 2014, 24, 5190.
Cui, H.-W.; He, Y.; Wang, J.; Gao, W.; Liu, T.; Qin, M.; Wang, X.; Gao, C.; Wang, Y.; Liu, M.-Y.; Yi, Z.; Qiu, W.-W. Eur. J. Med. Chem. 2015, 95, 240.
Babak, N. L.; Semenenko, A. N.; Gella, I. M.; Musatov, V. I.; Shishkina, S. V.; Novikova, N. B.; Sofronov, D. S.; Morina, D. A.; Lipson, V. V. Russ. J. Org. Chem. 2015, 51, 715. [Zh. Org. Khim. 2015, 51, 731.]
Khlebnicova, T. S.; Piven, Y. A.; Baranovsky, A. V.; Lakhvich, F. A.; Shishkina, S. V.; Zicāne, D.; Tetere, Z.; Rāviņa, I.; Kumpiņš, V.; Rijkure, I.; Mieriņa, I.; Peipiņš, U.; Turks, M. Steroids 2017, 117, 77.
Galaiko, N. V.; Nazarov, A. V.; Tolmacheva, I. A.; Slepukhin, P. A.; Vikharev, Yu. B.; Maiorova, O. A.; Grishko, V. V. Chem. Heterocycl. Compd. 2014, 50, 65. [Khim. Geterotsikl. Soedin. 2014, 72.]
Gubaidullin, R. R.; Yarmukhametova, D. S.; Nedopekina, D. A.; Khalitova, R. R.; Spivak, A. Yu. ARKIVOC 2017, (v), 100.
Grishko, V. V.; Tolmacheva, I. A.; Nebogatikov, V. O.; Galaiko, N. V.; Nazarov, A. V.; Dmitriev, M. V.; Ivshina, I. B. Eur. J. Med. Chem. 2017, 125, 629.
Zorina, A. D.; Nikiforova, N. S.; Zarubaev, V. V.; Marchenko, S. A.; Selivanov, S. I.; Starova, G. L.; Mehtiev, A. R.; Rodionov, E. I.; Rodionova A. A.; Trifonov, R. E. Mendeleev Commun. 2019, 29, 500.
Khusnutdinova, E. F.; Kazakova, O. B.; Lobov, A. N.; Kukovinets, O. S.; Suponitsky, K. Yu.; Meyers, C. B.; Prichard, M. N. Org. Biomol. Chem. 2019, 17, 585.
Borková, L.; Frydrych, I.; Jakubcová, N.; Adámek, R.; Lišková, B.; Gurská, S.; Medvedíková, M.; Hajdúch, M.; Urban, M. Eur. J. Med. Chem. 2019, 185, 111806.
Grishko, V. V.; Galaiko, N. V.; Tolmacheva, I. A.; Kucherov, I. I.; Eremin, V. F.; Boreko, E. I.; Savinova, O. V.; Slepukhin, P. A. Eur. J. Med. Chem. 2014, 83, 601.
Tolmacheva, I. A.; Galaiko, N. V.; Igosheva, E. V.; Konysheva, A. V.; Nazarov, A. V.; Krainova, G. F.; Gorbunova, M. N.; Boreko, E. I.; Eremin, V. F.; Grishko, V. V. In Chemistry and Technology of Plant Substances. Chemical and Biochemical Aspects; Kutchin, A. V.; Shishkina, L. N.; Weisfeld, L. I., Eds.; Apple Academic Press: Oakville, 2017, p. 3.
Konysheva, A. V.; Nebogatikov, V. O.; Tolmacheva, I. A.; Dmitriev, M. V.; Grishko, V. V. Eur. J. Med. Chem. 2017, 140, 74.
Tolmacheva, I. A.; Nazarov, A. V.; Eroshenko, D. V.; Grishko, V. V. Steroids 2018, 140, 131.
Tolmacheva, I. A.; Eroshenko, D. V.; Grishko, V. V. Chem. Nat. Compd. 2018, 54, 705. [Khim. Prirod. Soedin. 2018, 597.]
Klinot, J.; Světlý, J.; Kudlačková, D.; Buděšinský, M.; Vystrčil, A. Collect. Czech. Chem. Commun. 1979, 44, 211.
Nazarov, M. A.; Zhikina, L. A.; Tolmacheva, I. A.; Grishko, V. V. Bashk. Khim. Zhurn. [in Russian] 2017, 24(4), 28.
Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A. J. Appl. Crystallogr. 2008, 41, 466.
Lévai, A. J. Heterocycl. Chem. 2002, 39, 1.
Iványi, Z.; Szabó, N.; Huber, J.; Wölfling, J.; Zupkó, I.; Szécsi, M.; Wittmann, T.; Schneider, G. Steroids 2012, 77, 566.
Romero-López, A.; Montiel-Smith, S.; Meza-Reyes, S.; Merino-Montiel, P.; Vega-Baez, J. L. Steroids 2014, 87, 86.
Ghosh, P.; Mandal, A.; Ghosh, J.; Pal, C.; Nanda, A. K. J. Asian Nat. Prod. Res. 2012, 14, 141.
CrysAlisPro, Version 1.171.37.33 (release 27-03-2014 CrysAlis171.NET); Agilent Technologies: Yarnton, 2014.
Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Scudiero, D. A.; Shoemaker, R. H.; Paull, K. D.; Monks, A.; Tierney, S.; Notziger, T. H.; Currens, M. T.; Seniff, D.; Boyd, M. K. Cancer Res. 1988, 48, 4827.
This work was performed with financial support from the Russian Foundation for Basic Research (grant 18-03-00050).
The authors would like to express their gratitude to the Collective Use Center of Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences “Investigation of Materials and Compounds” for the spectral, analytic, and biological studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(10), 1321–1328
Supplementary Information
ESM 1
(PDF 2844 kb)
Rights and permissions
About this article
Cite this article
Nazarov, M.A., Tolmacheva, I.A., Eroshenko, D.V. et al. Synthesis of 1,2-azole derivatives on the basis of α,β-unsaturated triterpene aldehydes. Chem Heterocycl Comp 56, 1321–1328 (2020). https://doi.org/10.1007/s10593-020-02817-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02817-y