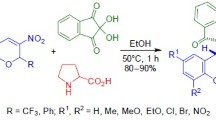
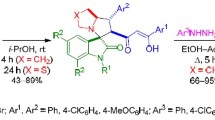
1,3-Dipolar cycloaddition of stabilized azomethine ylides generated in situ from isatins and proline to 3-nitro-2-phenyl-2-trifluoromethyl-2H-chromenes in i-PrOH proceeds stereoselectively at room temperature and leads to the formation of hexahydro-6H-spiro[chromeno[3,4-a]-pyrrolizine-11,3'-indolin]-2'-ones with the cis arrangement of the trifluoromethyl group and the nitro group. A similar reaction with the participation of thiaproline-based ylides at 50°С leads to mixtures of diastereomeric tetrahydro-6H,9H-spiro[chromeno[3',4':3,4]pyrrolo[1,2-c]thiazole-11,3'-indolin]-2'-ones with a predominance of the cis- or trans-isomer. The stereochemistry of the obtained products was confirmed by the NOESY experiment and X-ray structural analysis.





Similar content being viewed by others
References
(a) Vroemans, R.; Dehaen, W. In Targets in Heterocyclic Systems; Attanasi, O. A.; Merino, P.; Spinelli, D., Eds.; Società Chimica Italiana: Rome, 2018, Vol. 22, p. 318. (b) Korotaev, V. Yu.; Sosnovskikh, V. Ya.; Barkov, A. Yu. Russ. Chem. Rev. 2013, 82, 1081. [Usp. Khim. 2013, 82, 1081.] (с) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Russ. Chem. Rev. 2019, 88, 27. [Usp. Khim. 2019, 88, 27.] (d) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Rozhkova, Yu. S.; Plekhanova, I. V.; Shklyaev, Yu. V.; Sosnovskikh, V. Ya. Tetrahedron Lett. 2019, 60, 150916. d Soares, M. I. L.; Gomes, C. S. B.; Nunes, S. C. C.; Pais, A. A. C. C.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2019, 5441. e Dai, C.; Luo, N.; Wang, S.; Wang, C. Org. Lett. 2019, 21, 2828.
(a) Ito, M.; Egashira, S.-I.; Yoshida, K.; Mineno, T.; Kumagai, K.; Kojima, H.; Okabe, T.; Nagano, T.; Ui, M.; Matsuoka, I. Life Sci. 2017, 180, 137. (b) Tian, H.; Zhang, Y.; Zhang, Q.; Li, S.; Liu, Y.; Han, X. BioSci. Trends 2019, 13, 40. (c) Fouqué, A.; Delalande, O.; Jean, M.; Castellano, R.; Josselin, E.; Malleter, M.; Shoji, K. F.; Hung, M. D.; Rampanarivo, H.; Collette, Y.; van de Weghe, P.; Legembre, P. J. Med. Chem. 2015, 58, 6559. (d) Baral, N.; Mishra, D. R.; Mishra, N. P.; Mohapatra, S.; Raiguru, B. P.; Panda, P.; Nayak, S.; Nayak, M.; Kumar, P. S. J. Heterocycl. Chem. 2020, 57, 575. (e) Kutyashev, I. B.; Ulitko, M. V.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. New J. Chem. 2019, 43, 18495.
(a) Yu, B.; Yu, D.-Q.; Liu, H.-M. Eur. J. Med. Chem. 2015, 97, 673. (b) Zhao, Y.; Aguilar, A.; Bernard, D.; Wang, S. J. Med. Chem. 2015, 58, 1038. (c) Wang, S.; Sun, W.; Zhao, Y.; McEachern, D.; Meaux, I.; Barrière, C.; Stuckey, J. A.; Meagher, J. L.; Bai, L.; Liu, L.; Hoffman-Luca, C. G.; Lu, J.; Shangary, S.; Yu, S.; Bernard, D.; Aguilar, A.; Dos-Santos, O.; Besret, L.; Guerif, S.; Pannier, P.; Gorge-Bernat, D.; Debussche, L. Cancer Res. 2014, 74, 5855. (d) de Weger, V. A.; de Jonge, M.; Langenberg, M. H. G.; Schellens, J. H. M.; Lolkema, M.; Varga, A.; Demers, B.; Thomas, K.; Hsu, K.; Tuffal, G.; Goodstal, S.; Macé, S.; Deutsch, E. Br. J. Cancer 2019, 120, 286.
(a) Döndas, H. A.; Retamosa, M. G.; Sansano, J. M. Synthesis 2017, 2819. (b) Singh, M. S.; Chowdhury, S.; Koley, S. Tetrahedron 2016, 72, 1603. (c) Nájera, C.; Sansano, J. M. Pure Appl. Chem. 2019, 91, 575. (d) Korotaev, V. Yu.; Zimnitskiy, N. S.; Barkov, A. Yu.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 905. [Khim. Geterotsikl. Soedin. 2018, 54, 905.] (e) Pavlovska, T. L.; Redkin, R. G.; Lipson, V. V.; Atamanuk, D. V. Mol. Diversity 2016, 20, 299. (f) Lashgari, N.; Ziarani, G. M. ARKIVOC 2012, (i), 277. d Arumugam, N.; Kumar, R. S.; Almansour, A. I.; Perumal, S. Curr. Org. Chem. 2013, 17, 1929. e Izmest'ev, A. N.; Gazieva, G. А.; Kravchenko, A. N. Chem. Heterocycl. Compd. 2020, 56, 255. [Khim. Geterotsikl. Soedin. 2020, 56, 255.]
(a) Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2013, 54, 6568. (b) Rao, J. N. S.; Raghunathan, R. Tetrahedron Lett. 2015, 56, 2276. (c) Nayak, S.; Mishra, S. K.; Bhakta, S.; Panda, P.; Baral, N.; Mohapatra, S.; Purohit, C. S.; Satha, P. Lett. Org. Chem. 2016, 13, 11. (d) Nayak, S.; Pattanaik, P.; Mohapatra, S.; Mishra, D. R.; Panda, P.; Raiguru, B.; Mishra, N. P.; Jena, S.; Biswal, H. S. Synth. Commun. 2019, 49, 1823. (e) Nayak, S.; Panda, P.; Mohapatra, S.; Raiguru, B.; Baral, N. J. Heterocycl. Chem. 2019, 56, 1757. (f) Kutyashev, I. B.; Barkov, A. Yu.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2019, 55, 529. [Khim. Geterotsikl. Soedin. 2019, 55, 529.] (g) Kutyashev, I. B.; Barkov, A. Yu.; Zimnitskiy, N. S.; Korotaev, V. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2019, 55, 861. [Khim. Geterotsikl. Soedin. 2019, 55, 861.]
(a) Habib, P. M.; Raju, B. R.; Kavala, V.; Kuo, C.-W.; Yao, C.-F. Tetrahedron 2009, 65, 5799. (b) Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2017, 53, 597. [Khim. Geterotsikl. Soedin. 2017, 53, 597.]
Korotaev, V. Yu.; Kutyashev, I. B.; Barkov, A. Yu.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2018, 54, 852. [Khim. Geterotsikl. Soedin. 2018, 54, 852.]
Barkov, A. Yu.; Korotaev, V. Yu.; Kotovich, I. V.; Zimnitskiy, N. S.; Kutyashev, I. B.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd. 2016, 52, 814. [Khim. Geterotsikl. Soedin., 52, 814.]
(a) Toma, Y.; Kunigami, M.; Watanabe, K.; Higashi, M.; Arimitsu, S. J. Fluorine Chem. 2016, 189, 22. (b) Grigg, R.; Idle, J.; McMeekin, P.; Surendrakumar, S.; Vipond, D. J. Chem. Soc., Perkin Trans. 1 1988, 2703.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2015, A71, 3.
This work was carried out with the financial support of the Russian Foundation for Basic Research (project 20-03-00716) and within the framework of the State Assignment of the Ministry of Science and Higher Education of the Russian Federation (project FEUZ-2020-0052).
The authors are grateful to the staff of the Center for Collective Use “Spectroscopy and Analysis of Organic Compounds” of Postovsky Institute of Organic Synthesis of the Ural Branch of the Russian Academy of Sciences and the Laboratory for Complex Research and Expert Evaluation of Organic Materials of Ural Federal University for assistance in carrying out physicochemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(10), 1302–1313
Rights and permissions
About this article
Cite this article
Kutyashev, I.B., Kochnev, I.А., Cherepkova, A.А. et al. 3-Nitro-2-phenyl-2-trifluoromethyl-2H-chromenes in reactions with azomethine ylides from isatins and (thia)proline: synthesis of spiro[chromeno(thia)pyrrolizidine-11,3'-oxindoles]. Chem Heterocycl Comp 56, 1302–1313 (2020). https://doi.org/10.1007/s10593-020-02815-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02815-0