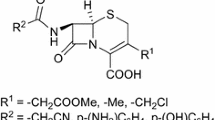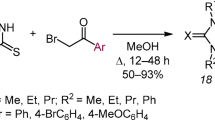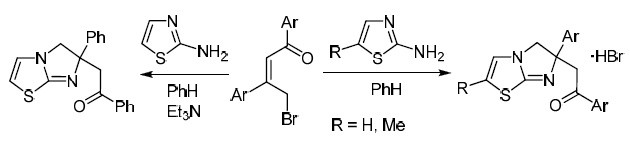
We propose a new method for assembling the imidazo[2,1-b][1,3]thiazole system, based on the reaction of (2Z)-1,3-diaryl-4-bromobut-2-en-1-one derivatives with 2-aminothiazoles. The outcome of this reaction depends on the structure of the starting bromo ketone: when electron-withdrawing substituents are present in the structure of the ketone, a competing reaction occurs, which leads to the formation of 2,5-diarylfurans. Screening for antitumor activity has been performed in the case of 1-phenyl-2-(6-phenyl-5,6-dihydroimidazo[2,1-b]- thiazol-6-yl)ethanone and this compound has shown moderate ability to suppress the growth of kidney cancer cells, with a weaker effect on the cell lines of prostate cancer, colon cancer, and leukemia.

Similar content being viewed by others
References
Buron, F.; Hiebel, M.-A.; Mérour, J.-Y.; Plé, K.; Routier, S. In Advances in Heterocyclic Chemistry; Katritzky, A. R., Ed.; Elsevier: New York, 2018, Vol. 125, p. 301.
Kamal, A.; Reddy, M. K.; Viswanath, A. Expert Opin. Drug Discovery 2013, 8, 289.
(а) Amarouch, H.; Loiseau, P. R.; Bonnafous, M.; Caujolle, R.; Payard, M.; Loiseau, P. M.; Bories, C.; Gayral, P. Farmaco, Ed. Sci. 1988, 43, 421. a Gomha, S. M.; Edrees, M. M.; El- Arab, E. E. J. Heterocycl. Chem. 2017, 54 , 641. b Miyazaki, M.; Naito, H.; Sugimoto, Y.; Kawato, H.; Okayama, T.; Shimizu, H.; Miyazaki, M.; Kitagawa, M.; Seki, T.; Fukutake, S.; Aonuma, M.; Soga, T. Bioorg. Med. Chem. Lett. 2013, 23, 728. c Mona, C. E.; Besserer-Offroy, É.; Cabana, J.; Leduc, R.; Lavigne, P.; Heveker, N.; Marsault, É.; Escher, E. Org. Biomol. Chem. 2016, 14, 10298. d Thoma, G.; Streiff, M. B.; Kovarik, J.; Glickman, F.; Wagner, T.; Beerli, C.; Zerwes, H.-G. J. Med. Chem. 2008, 51, 7915. e Li, Y.; Bionda, N.; Fleeman, R.; Wang, H.; Ozawa, A.; Houghten, R. A.; Shaw, L. Bioorg. Med. Chem. 2016, 24, 5633. f Yallur, B. C.; Katrahalli, U.; Krishna, P. M.; Hadagali, M. D. Spectrochim. Acta, Part A 2019, 222, 117192.
(a) Okamoto, S.; Sakai, Y.; Watanabe, S.; Nishi, S.; Yoneyama, A.; Katsumata, H.; Kosaki, Y.; Sato, R.; Shiratori, M.; Shibuno, M.; Shishido, T. Tetrahedron Lett. 2014, 55, 1909. (b) Ahlemeyer, N. A.; Streff, E. V.; Muthupandi, P.; Birman, V. B. Org. Lett. 2017, 19, 6486.
Kovtunenko, V.; Potikha, L.; Turov, A. Synth. Commun. 2004, 34, 3609.
Potikha, L. M. Fr.-Ukr. J. Chem. 2018, 6, 56.
Jiang, H.; Zeng, W.; Li, Y.; Wu, W.; Huang, L.; Fu, W. J. Org. Chem. 2012, 77, 5179.
(a) Alley, M. C.; Scudiero, D. A.; Monks, A.; Hursey, M. L.; Czerwinski, M. J.; Fine, D. L.; Abbott, B. J.; Mayo, J. G.; Shoemaker, R. H.; Boyd, M. R. Cancer Res. 1988, 48, 589. (b) Grever, M. R.; Schepartz, S. A.; Chabner, B. A. Semin. Oncol. 1992, 19, 622. (c) Boyd, M. R. and Paull, K. D. Drug Dev. Res. 1995, 34, 91. (d) Shoemaker, R. H. Nat. Rev. Cancer 2006, 6, 813.
(a) Wasserman, H. H.; Aubrey, N. E., Zimmerman, H. E. J. Am. Chem. Soc. 1953, 75, 96. (b) Potikha, L. M.; Turelik, A. R.; Kovtunenko, V. A. Chem. Heterocycl. Compd. 2009, 45, 1184. [Khim. Geterotsikl. Soedin. 2009, 1478.] (с) Van Tamalen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1968, 90, 3894.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(8), 1073–1077
Electronic supplementary material
ESM 1
(PDF 1225 kb)
Rights and permissions
About this article
Cite this article
Potikha, L.M., Brovarets, V.S. Synthesis of imidazo[2,1-b][1,3]thiazoles – potential anticancer agents derived from γ-bromodipnones. Chem Heterocycl Comp 56, 1073–1077 (2020). https://doi.org/10.1007/s10593-020-02776-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02776-4