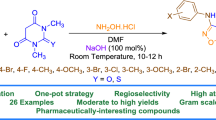
A new multicomponent reaction for regioselective synthesis of substituted 1,2,4-triazolo[1,5-a]pyrimidines using aminotriazoles, β-ketoglutaric acid dimethyl ester, and dimethylformamide dimethyl acetal was implemented. The selective reduction of 1,2,4-triazolo-[1,5-a]pyrimidines to dihydro derivatives and the use of triazolo pyrimidines as starting compounds in multicomponent synthesis of dihydropyrido[3,4-е][1,2,4]triazolo[1,5-a]pyrimidinecarboxylates was demonstrated.


Similar content being viewed by others
References
(a) Singh, P. K.; Choudhary, S.; Kashyap, A.; Verma, H.; Kapil, S.; Kumar, M.; Arora, M.; Silakari, O. Bioorg. Chem. 2019, 88, 102919. (b) Fischer, G. Adv. Heterocycl. Chem. 2019, 128, 1. (c) Rusinov, V. L.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed. 2018, 67, 573. [Izv. Akad. Nauk, Ser. Khim. 2018, 573.]
Ribeiro, C. J. A.; Kankanala, J.; Xie, J.; Williams, J.; Aihara, H.; Wang, Z. Bioorg. Med. Chem. Lett. 2019, 29, 257.
Singh, P. K.; Chaudhari, D.; Jain, S.; Silakari, O. Bioorg. Med. Chem. Lett. 2019, 29, 1565.
Wang, S.; Li, Z.-R.; Suo, F.-Z.; Yuan, X.-H.; Yu, B.; Liu, H.-M. Eur. J. Med. Chem. 2019, 167, 388.
Wang, H.; Lee, M.; Peng, Z.; Blázquez, B.; Lastochkin, E.; Kumarasiri, M.; Bouley, R.; Chang, M.; Mobashery, S. J. Med. Chem. 2015, 58, 4194.
Said, S. A.; Amr, A. E.-G.; Sabry, N. M.; Abdalla, M. M. Eur. J. Med. Chem. 2009, 44, 4787.
Lahmidi, S.; Anouar, E. H.; El Hafi, M.; Boulhaoua, M.; Ejjoummany, A.; El Jemli, M.; Essassi, El. M.; Mague, J. T. J. Mol. Struct. 2019, 1177, 131.
Gilandoust, M.; Harsha, K. B.; Mohan, C. D.; Raquib, A. R.; Rangappa, S.; Pandey, V.; Lobie, P. E.; Basappa; Rangappa K. S. Bioorg. Med. Chem. Lett. 2018, 28, 2314.
Ding, J.; Cao F.-D.; Geng Y.-R.; Tian Y.; Li P.; Li X.-F.; Huang L.-J. J. Asian Nat. Prod. Res. 2018, 21, 1190.
Monti, L.; Wang, S. C.; Oukoloff, K.; Smith III, A. B.; Brunden, K. R.; Caffrey, C. R.; Ballatore, C. ChemMedChem 2018, 13, 1751.
El-Gendy, M. M.; Shaaban, M.; Shaaban, K. A.; El- Bondkly, A. M.; Laatsch, H. J. Antibiot. 2008, 61, 149.
Büyükafşar, K.; Yazar, A.; Düşmez, D.; Öztürk, H.; Polat, G.; Levent, A. Pharmacol. Res. 2001, 44, 321.
Sato, Y.; Shimoji, Y.; Fujita, H.; Nishino, H.; Mizuno, H.; Kobayashi, S.; Kumakura, S. J. Med. Chem. 1980, 23, 927.
Hassan, G. S.; El-Sherbeny, M. A.; El-Ashmawy, M. B.; Bayomi, S. M.; Maarouf, A. R.; Badria, F. A. Arabian J. Chem. 2017, 10, S1345.
Farghaly, T. A.; Dawood, K. M.; Shaaban, M. R. Curr. Org. Synth. 2015, 12, 230.
Lahmidi, S.; Elyoussfi, A.; Dafali, A.; Elmsellem, H.; Sebbar, N. K.; El Ouasif, L.; Jilalat, A.E.; El Mahi, B.; Essassi, E. M.; Abdel-Rahman, I.; Hammouti, B. J. Mater. Environ. Sci. 2017, 8, 225.
Fizer, M.; Slivka, M. Chem. Heterocycl. Compd. 2016, 52, 155. [Khim. Geterotsikl. Soedin. 2016, 52, 155.]
Chebanov, V. A.; Gura, K. A.; Desenko, S. M. Top. Heterocycl. Chem. 2010, 23, 41.
(a) Akrami, S.; Karami, B.; Farahi, M. Mol. Diversity 2019, 24, 225.(b) Lyapustin, D. N.; Ulomsky, E. N.; Voinkov, E. K.; Rusinov, V. L. AIP Conf. Proc. 2019, 2063, 030012. (c) Baklykov, A. V.; Rusinov, G. L.; Artem'ev, G. A.; Kopchuk, D. S.; Zyryanov, G. V.; Rusinov, V. L.; Charushin, V. N. AIP Conf. Proc. 2019, 2063, 040005. (d) Astakhov, A. V.; Suponitsky, K. Y.; Chernyshev, V. M. Mendeleev Commun. 2018, 28, 439. (e) Souza, L. A.; Santos, J. M.; Mittersteiner, M.; Andrade, V. P.; Lobo, M. M.; Santos, F. B.; Bortoluzzi, A. J.; Bonacorso, H. G.; Martins, M. A. P.; Zanatta, N. Synthesis 2018, 3686. (f) Alnajjar, A.; Abdelkhalik, M. M.; Raslan, M. A.; Ibraheem, S. M.; Sadek, K. U. J. Heterocycl. Chem. 2018. 55, 1804. (g) Prezent, M. A.; Daeva, E. D.; Baranin, S. V.; Zavarzin, I. V. Mendeleev Commun. 2017, 27, 169. (h) Titova, Yu. A.; Fedorova, O. V.; Rusinov, G. L.; Charushin, V. N. Russ. Chem. Bull., Int. Ed. 2019, 68, 2271. [Izv. Akad. Nauk, Ser. Khim. 2019, 2271.]
(a) Kolosov, M. A.; Shvets, E. H.; Manuenkov, D. A.; Vlasenko, S. A.; Omelchenko, I. V.; Shishkina, S. V.; Orlov, V. D. Tetrahedron Lett. 2017, 58, 1207. (b) Gümüş, M. K.; Gorobets, N. Yu.; Sedash, Y. V.; Shishkina, S. V.; Desenko, S. M. Tetrahedron Lett. 2017, 58, 3446.
(a) Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566. [Khim. Geterotsikl. Soedin. 2012, 607.] (b) Chechina, N. V.; Kolos, N. N.; Omelchenko, I. V.; Musatov, V. I. Chem. Heterocycl. Compd. 2018, 54, 58. [Khim. Geterotsikl. Soedin. 2018, 54, 58.]
(a) Voskressensky, L. G.; Borisova, T. N; Ovcharov, M. V.; Sorokina, E. A.; Khrustalev, V. N.; Varlamov, A. V. Russ. Chem. Bull., Int. Ed. 2012, 61, 1603. [Izv. Akad. Nauk, Ser. Khim. 2012, 1587.] (b) Farahi, M.; Karami, B.; Banaki, Z. Chin. Chem. Lett. 2015, 26, 1065.
(a) Shikhaliev, K. S.; Potapov. A. Y.; Polukhin, E. L.; Slivkin, A. I. Russ. Chem. Bull., Int. Ed. 2009, 58, 1996. [Izv. Akad. Nauk, Ser. Khim. 2009, 1934.] (b) Didenko, V. V.; Potapov, A. Y.; Ledeneva, I. V.; Shikhaliev, K. S.; Konyushko, O. V. Russ. J. Gen. Chem. 2010, 80, 814. [Zh. Obshch. Khim. 2010, 80, 653.]
(a) Robinson, R. J. Chem. Soc., Trans. 1917, 111, 762. (b) Stoll, A.; Jucker, E.; Lindenmann, A. Helv. Chim. Acta 1954, 37, 649. (c) Latypova, D. R.; Vlasova, L. I.; Baibulatova, N. Z.; Lobov, A. N.; Spirikhin, L. V.; Dokichev, V. A. Chem. Heterocycl. Compd. 2008, 44, 996. [Khim. Geterotsikl. Soedin. 2008, 1236.]
(a) Stanovnik, B.; Grošelj, U. Adv. Heterocycl. Chem. 2010, 100, 145. (b) Francis, S.; Croft, D.; Schüttelkopf, A. W.; Parry, C.; Pugliese, A.; Cameron, K.; Claydon, S.; Drysdale, M.; Gardner, C.; Gohlke, A.; Goodwin, G.; Gray, C. H.; Konczal, J.; McDonald, L.; Mezna, M.; Pannifer, A.; Paul, N. R.; Machesky, L.; McKinnon, H.; Bower J. Bioorg. Med. Chem. Lett. 2019, 29, 1023. (c) Satheesh, M.; Balachandran, A. L.; Devi, P. R.; Deepthi, A. Synth. Commun. 2018, 48, 582. (d) Li, W.; Ruzi, R.; Ablajan, K.; Ghalipt, Z. Tetrahedron 2017, 73, 164. (e) Grošelj, U.; Pušavec, E.; Golobič, A.; Dahmann, G.; Stanovnik, B.; Svete, J. Tetrahedron 2015, 71, 109. e Koohshari, M.; Dabiri, M.; Salehi, P. RSC Adv. 2014, 4, 10669. f Akbari, A.; Azami-Sardooei, Z.; Hosseini-Nia, A. J. Korean Chem. Soc. 2013, 57, 455. g Šporar, J.; Bezenšek, J.; Uršič, U.; Golobič, A.; Svete, J.; Stanovnik, B. Heterocycles 2012, 84, 449.
(a) Bevk, D.; Grošelj, U.; Meden, A.; Svete, J.; Stanovnik, B. Helv. Chim. Acta 2007, 90, 1737. (b) Zupancic, S.; Svete, J.; Stanovnik, B. Heterocycles 2009, 77, 899. (c) Svĕtlík, J.; Veizerová, L.; Kettmann, V. Tetrahedron Lett. 2008, 49, 3520. (d) Azizian, J.; Mohammadi, A. A.; Kohshari, M.; Karimi, A. R.; Mohammadizadeh, M. R. J. Heterocycl. Chem. 2007, 44, 455.
Haasnoot, J. G. Coord. Chem. Rev. 2000, 200-202, 131.
(a) Desenko, S. M.; Shishkin, O. V.; Orlov, V. D.; Lipson, V. V.; Lindeman, S. V.; Struchkov, Yu. T. Chem. Heterocycl. Compd. 1994, 30, 851. [Khim. Geterotsikl. Soedin. 1994, 981.] (b) Chebanov, V. A.; Muravyova, E. A.; Desenko S. M.; Musatov V. I.; Knyazeva, I. V.; Shishkina, S. V.; Shishkin, O. V.; Kappe, C. O. J. Comb. Chem. 2006, 8, 427. a Chernyshev, V. M.; Sokolov, A. N.; Khoroshkin, D. A.; Taranushich, V. A. Russ. J. Org. Chem. 2008, 44, 715. [Zh. Org. Khim. 2008, 44, 724].
Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444.
Desenko, S. М; Orlov, V. D. Azageterotsikly na osnove aromaticheskikh nepredel'nykh ketonov (Azaheterocycles Based on Aromatic Unsaturated Ketones [in Russian]); Kharkiv: Folio, 1998, p. 122.
The study was carried out with a grant from the Russian Science Foundation (project No. 18-74-10097).
The results of the study were partially obtained using the equipment of the Center for Collective Use of the Voronezh State University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(8), 1054–1061
Electronic supplementary material
ESM 1
(PDF 26538 kb)
Rights and permissions
About this article
Cite this article
Polikarchuk, V.А., Chertova, Y.V., Potapov, A.Y. et al. Novel variants of the multicomponent reaction for the synthesis of 1,2,4-triazolo[1,5-а]pyrimidines and pyrido[3,4-е][1,2,4]triazolo[1,5-а]pyrimidines. Chem Heterocycl Comp 56, 1054–1061 (2020). https://doi.org/10.1007/s10593-020-02773-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02773-7