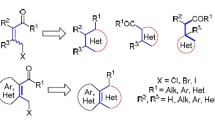
Being compounds with multiple electrophilic centers, cross-conjugated enynones can be involved in various transformations upon treatment with electrophiles, in particular, Lewis and Brønsted acids, providing a convenient access to acyclic and cyclic compounds. This review covers literature data on electrophile-assisted reactions of substrates with cross-conjugated enynone scaffold toward the synthesis of heterocyclic compounds. The information is arranged according to the type of enynone motif, and chemical transformations of 2-(alkyn-1-yl)alk-2-en-1-ones and alk-1-en-4-yn-3-ones are discussed. The bibliography includes 52 sources.

Similar content being viewed by others
References
For recent reviews, see: (a) Golovanov, A. A.; Odin, I. S.; Zlotskii, S. S. Russ. Chem. Rev. 2019, 88, 280. [Usp. Khim. 2019, 88, 280.] (b) Golovanov, A. A.; Gusev, D. M.; Odin, I. S.; Zlotskii, S. S. Chem. Heterocycl. Compd. 2019, 55, 333. [Khim. Geterotsikl. Soedin. 2019, 55, 333.] (c) Chen, L.; Liu, Z.; Zhu, S. Org. Biomol. Chem. 2018, 16, 8884.
For our group's work on this topic, see: (a) Golubev, P. R.; Pankova, A. S.; Kuznetsov, M. A. J. Org. Chem. 2015, 80, 4545. a Golubev, P.; Karpova, E. A.; Pankova, A. S.; Sorokina, M.; Kuznetsov, M. A. J. Org. Chem. 2016, 81, 11268. b Pankova, A. S.; Golubev, P. R.; Ananyev, I. V.; Kuznetsov, M. A. Eur. J. Org. Chem. 2012, 5965. c Golubev, P. R.; Pankova, A. S.; Kuznetsov, M. A. Eur. J. Org. Chem. 2014, 3614.
(a) Siva Kumari, A. L.; Siva Reddy, A.; Kumara Swamy, K. C. Org. Biomol. Chem. 2016, 14, 6651. (b) Asao, N. Synlett 2006, 1645. (c) Chen, L.; Chen, K.; Zhu, S. Chem 2018, 4, 1208.
(a) Zhu, D.; Chen, L.; Fan, H.; Yao, Q.; Zhu, S. Chem. Soc. Rev. 2020, 49, 908. (b) Ma, J.; Zhang, L.; Zhu, S. Curr. Org. Chem. 2016, 20, 102.
Tandon, V. K.; Singh, K. A. Chem. Biol. Interface 2012, 2, 76.
Yao, T.; Zhang, X.; Larock, R. C. J. Am. Chem. Soc. 2004, 126, 11164.
Yao, T.; Zhang, X.; Larock, R. C. J. Org. Chem. 2005, 70, 7679.
(a) Cho, C.-H.; Larock, R. C. ACS Comb. Sci. 2011, 13, 272. (b) Cho, C.-H.; Shi, F.; Jung, D.-I.; Neuenswander, B.; Lushington, G. H.; Larock, R. C. ACS Comb. Sci. 2012, 14, 403.
(a) He, T.; Chi, Y.; Chen, Y. Chem. Pap. 2018, 72, 691. (b) Chen, W.-L.; Li, J.; Zhu, Y.-H.; Ye, L.-T.; Hu, W.; Mo, W.-M. ARKIVOC 2011, (ix), 381.
Li, W.; Zhang, J. Chem. Commun. 2010, 46, 8839.
Zhou, G; Zhang, J. Chem. Commun. 2010, 46, 6593.
Zhou, G.; Liu, F.; Zhang, J. Chem.–Eur. J. 2011, 17, 3101.
Liu, F.; Yu, Y.; Zhang, J. Angew. Chem., Int. Ed. 2009, 48, 5505.
Liu, F.; Qian, D.; Li, L.; Zhao, X.; Zhang, J. Angew. Chem., Int. Ed. 2010, 49, 6669.
Zhang, Z.-M.; Chen, P.; Li, W.; Niu, Y.; Zhao, X.-L.; Zhang, J. Angew. Chem., Int. Ed. 2014, 53, 4350.
Du, Q.; Neudoerfl, J.-M.; Schmalz, H.-G. Chem.–Eur. J. 2018, 24, 2379.
Siva Kumari, A. L.; Kumara Swamy, K. C. J. Org. Chem. 2016, 81, 1425.
Wang, Y.; Zhang, P.; Qian, D.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 14849.
Pathipati, S. R.; van der Werf, A.; Eriksson, L.; Selander, N. Angew. Chem., Int. Ed. 2016, 55, 11863.
Qi, J.; Teng, Q.; Thirupathi, N.; Tung, C.-H.; Xu, Z. Org. Lett. 2019, 21, 692.
He, T.; Gao, P.; Qiu, Y.-F.; Yan, X.-B.; Liu, X.-Y.; Liang, Y.-M. RSC Adv. 2013, 3, 19913.
He, T.; Gao, P.; Zhao, S.-C.; Shi, Y.-D.; Liu, X.-Y.; Liang, Y.-M. Adv. Synth. Catal. 2013, 355, 365.
Krafft, M. E.; Vidhani, D. V.; Cran, J. W.; Manoharan, M. Chem. Commun. 2011, 47, 6707.
Gao, H.; Zhang, J. Chem.–Eur. J. 2012, 18, 2777.
Gao, H.; Zhao, X.; Yu, Y.; Zhang, J. Chem.–Eur. J. 2010, 16, 456.
Gao, H.; Wu, X.; Zhang, J. Chem.–Eur. J. 2011, 17, 2838.
Wang, Y.; Zhang, Z.-M.; Liu, F.; He, Y.; Zhang, J. Org. Lett. 2018, 20, 6403.
Di, X.; Wang, Y.; Wu, L.; Zhang, Z.-M.; Dai, Q.; Li, W.; Zhang, J. Org. Lett. 2019, 21, 3018.
Gao, H.; Wu, X.; Zhang, J. Chem. Commun. 2010, 46, 8764.
(a) Zheng, Y.; Chi, Y.; Bao, M.; Qiu, L.; Xu, X. J. Org. Chem. 2017, 82, 2129. (b) Liu, S.; Yang, P.; Peng, S.; Zhu, C.; Cao, S.; Li, J.; Sun, J. Chem. Commun. 2017, 53, 1152.
Zhao, W.; Zhang, J. Chem. Commun. 2010, 46, 4384.
Zhao, W.; Zhang, J. Chem. Commun. 2010, 46, 7816.
Zhao, W.; Zhang, J. Org. Lett. 2011, 13, 688.
Liu, R.; Zhang, J. Adv. Synth. Catal. 2011, 353, 36.
Liu, R.; Zhang, J. Chem. Asian J. 2012, 7, 294.
Wang, T.; Shi, S.; Hansmann, M. M.; Rettenmeier, E.; Rudolph, M.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2014, 53, 3715.
Heffernan, S. J.; Tellam, J. P.; Queru, M. E.; Silvanus, A. C.; Benito, D.; Mahon, M. F.; Hennessy, A. J.; Andrews, B. I.; Carbery, D. R. Adv. Synth. Catal. 2013, 355, 1149.
Xie, X.; Du, X.; Chen, Y.; Liu, Y. J. Org. Chem. 2011, 76, 9175.
Ciesielski, J.; Canterbury, D. P.; Frontier, A. J. Org. Lett. 2009, 11, 4374.
Ciesielski, J.; Laboeuf, D.; Stern, H. A.; Frontier, A. J. Adv. Synth. Catal. 2013, 355, 2077.
Saulnier, S.; Lozovskiy, S. V.; Golovanov, A. A.; Ivanov, A. Yu.; Vasilyev, A. V. Eur. J. Org. Chem. 2017, 3635.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(7), 829–836
Rights and permissions
About this article
Cite this article
Pankova, A.S. Electrophile-Induced Reactions of Cross-Conjugated Enynones in the Synthesis of Heterocycles. Chem Heterocycl Comp 56, 829–836 (2020). https://doi.org/10.1007/s10593-020-02739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02739-9