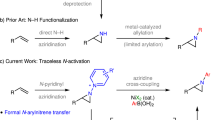
The review is devoted to the reactions of azine N-oxides with electrophilic reagents, which make it possible to functionalize the azine fragment with the formation of a new C–X bond (X = C, N, O, Hal, S, P) without the participation of transition metal complexes. The use of azine N-oxides as starting compounds is often associated with the employment of electrophilic reagents or media. The reaction of electrophiles with azine N-oxides allows one to purposefully and selectively introduce substituents at positions 2, 3, or 4 of the azine fragment (relative to the nitrogen atom), depending on the choice of the reagent or the reaction conditions. The review considers the reactions of intramolecular nucleophilic substitution with preliminary generation of azine N-oxide adducts with electrophilic reagents, SEAr reactions, and photocatalytic reactions. Original research published over the past 5 years is covered.


Similar content being viewed by others
References
Albini, A.; Pietra, S. Heterocyclic N-Oxides; CRC Press: Boca Raton, 1991.
Wang, Y.; Zhang, L. Synthesis 2015, 289.
(a) Koukal, P.; Ulč, J.; Nečas, D.; Kotora, M. Top. Heterocycl. Chem. 2017, 53, 29. (b) Yeom, H.-S.; Shin, S. Acc. Chem. Res. 2014, 47, 966.
Stephens, D. E.; Larionov, O. V. Top. Heterocycl. Chem. 2017, 53, 59.
Loska, R. Top. Heterocycl. Chem. 2017, 53, 85.
Poole, J. S. Top. Heterocycl. Chem. 2017, 53, 111.
Kouznetsov, V. V.; Vargas Méndez, L. Y.; Puerto Galvis, C. E.; Ortiz Villamizar, M. C. New J. Chem. 2020, 44, 12.
Kumar, R.; Kumar, I.; Sharma, R.; Sharma, U. Org. Biomol. Chem. 2016, 14, 2613.
Crisenza, G. E. M.; Dauncey, E. M.; Bower, J. F. Org. Biomol. Chem. 2016, 14, 5820.
Xia, H.; Liu, Y.; Zhao, P.; Gou, S.; Wang, J. Org. Lett. 2016, 18, 1796.
Zhang, B.; Huang, L.; Yin, S.; Li, X.; Xu, T.; Zhuang, B.; Wang, T.; Zhang, Z.; Hashmi, A. S. K. Org. Lett. 2017, 19, 4327.
Li, X.; Wang, T.; Zhang, Z. Adv. Synth. Catal. 2019, 361, 696.
Zhang, S.; Wu, C.; Zhang, Z.; Wang, T. Org. Lett. 2019, 21, 9995.
Dhiman, A. K.; Kumar, R.; Kumar, R.; Sharma, U. J. Org. Chem. 2017, 82, 12307.
Lv, C.; Wan, C.; Liu, S.; Lan, Y.; Li, Y. Org. Lett. 2018, 20, 1919.
Chen, X.; Ruider, S. A.; Hartmann, R. W.; González, L.; Maulide, N. Angew. Chem., Int. Ed. 2016, 55, 15424.
Childress, E. S.; Wieting, J. M.; Felts, A. S.; Breiner, M. M.; Long, M. F.; Luscombe, V. B.; Rodriguez, A. L.; Cho, H. P.; Blobaum, A. L.; Niswender, C. M.; Emmitte, K. A.; Conn, P. J.; Lindsley, C. W. J. Med. Chem. 2019, 62, 378.
Maiwald, M. M.; Wagner, A. T.; Kratsch, J.; Skerencak- Frech, A.; Trumm, M.; Geist, A.; Roesky, P. W.; Panak, P. J. Dalton Trans. 2017, 46, 9981.
Liu, F.-W.; Bi, J.; Sun, Y.; Luo, S.; Kang, P. ChemSusChem 2018, 11, 1656.
Sarmah, B. K.; Konwar, M.; Bhattacharyya, D.; Adhikari, P.; Das, A. Adv. Synth. Catal. 2019, 361, 5616.
Puthanveedu, M.; Polychronidou, V.; Antonchick, A. P. Org. Lett. 2019, 21, 3407.
Bering, L.; Antonchick, A. P. Org. Lett. 2015, 17, 3134.
Sen, C.; Ghosh, S. C. Adv. Synth. Catal. 2018, 360, 905.
Harisha, M. B.; Nagaraj, M.; Muthusubramanian, S.; Bhuvanesh, N. RSC Adv. 2016, 6, 58118.
Dhiman, A. K.; Chandra, D.; Kumar, R.; Sharma, U. J. Org. Chem. 2019, 84, 6962.
Zhang, Q.-W.; Hartwig, J. F. Chem. Commun. 2018, 10124.
Fumagalli, F.; da Silva Emery, F. J. Org. Chem. 2016, 81, 10339.
Xie, L.-Y.; Li, Y.-J.; Qu, J.; Duan, Y.; Hu, J.; Liu, K.-J.; Cao, Z.; He, W.-M. Green Chem. 2017, 19, 5642.
Wang, H.; Cui, X.; Pei, Y.; Zhang, Q.; Bai, J.; Wei, D.; Wu, Y. Chem. Commun. 2014, 14409.
Neelakantan, H.; Wang, H.-Y.; Vance, V.; Hommel, J. D.; McHardy, S. F.; Watowich, S. J. J. Med. Chem. 2017, 60, 5015.
Penjarla, T. R.; Kundarapu, M.; Baquer, S. M.; Bhattacharya, A. ChemistrySelect 2018, 3, 5386.
Smets, R. J.; Torfs, E.; Lemière, F.; Cos, P.; Cappoen, D.; Abbaspour Tehrani, K. Org. Biomol. Chem. 2019, 17, 2923.
Wang, D.; Jia, H.; Wang, W.; Wang, Z. Tetrahedron Lett. 2014, 55, 7130.
Wang, D.; Wang, Y.; Zhao, J.; Li, L.; Miao, L.; Wang, D.; Sun, H.; Yu, P. Tetrahedron 2016, 72, 5762.
Qian, W.; Brown, J.; Chen, J. J.; Cheng, Y. Tetrahedron Lett. 2014, 55, 7229.
Qiao, K.; Wan, L.; Sun, X.; Zhang, K.; Zhu, N.; Li, X.; Guo, K. Eur. J. Org. Chem. 2016, 1606.
Kijrungphaiboon, W.; Chantarasriwong, O.; Chavasiri, W. Tetrahedron Lett. 2012, 53, 674.
Chen, Y.; Huang, J.; Hwang, T.-L.; Chen, M. J.; Tedrow, J. S.; Farrell, R. P.; Bio, M. M.; Cui, S. Org. Lett. 2015, 17, 2948.
Han, C.; Green, K.; Pfeifer, E.; Gosselin, F. Org. Process Res. Dev. 2017, 21, 664.
González-Bobes, F.; Hickey, M. R.; Cohen, B.; Bultman, M.; Chen, K.; Fanfair, D.; Rosso, V. W.; Strotman, N. A.; Mudryk, B.; Murugesan, S.; Schild, R. L.; Ivy, S.; Eastgate, M. D.; Sweeney, J. T.; Conlon, D. A. Org. Process Res. Dev. 2017, 21, 1137.
Prokhorov, A. M.; Kozhevnikov, D. N.; Rusinov, V. L.; Chupakhin, O. N. Russ. Chem. Bull., Int. Ed. 2003, 52, 1195.
Rassadin, V. A.; Boyarskiy, V. P.; Kukushkin, V. Y. Org. Lett. 2015, 17, 3502.
Rassadin, V. A.; Zimin, D. P.; Raskil'dina, G. Z.; Ivanov, A. Y.; Boyarskiy, V. P.; Zlotskii, S. S.; Kukushkin, V. Y. Green Chem. 2016, 18, 6630.
Geyl, K.; Baykov, S.; Tarasenko, M.; Zelenkov, L. E.; Matveevskaya, V.; Boyarskiy, V. P. Tetrahedron Lett. 2019, 60, 151108.
Chen, X.; Peng, M.; Huang, H.; Zheng, Y.; Tao, X.; He, C.; Xiao, Y. Org. Biomol. Chem. 2018, 16, 6202.
Xie, L.-Y.; Peng, S.; Lu, L.-H.; Hu, J.; Bao, W.-H.; Zeng, F.; Tang, Z.; Xu, X.; He, W.-M. ACS Sustain. Chem. Eng. 2018, 6, 7989.
Kruck, C.; Nazari, P.; Dee, C.; Richards, B. S.; Turshatov, A.; Seitz, M. Inorg. Chem. 2019, 58, 6959.
Karpacheva, M.; Malzner, F. J.; Wobill, C.; Büttner, A.; Constable, E. C.; Housecroft, C. E. Dyes Pigments 2018, 156, 410.
Shimoda, T.; Morishima, T.; Kodama, K.; Hirose, T.; Polyansky, D. E.; Manbeck, G. F.; Muckerman, J. T.; Fujita, E. Inorg. Chem. 2018, 57, 5486.
Gavriil, E.-S.; Doukatas, A.; Karampelas, T.; Myrianthopoulos, V.; Dimitrakis, S.; Mikros, E.; Marakos, P.; Tamvakopoulos, C.; Pouli, N. Eur. J. Med. Chem. 2019, 176, 393.
Yang, Y.; Yang, F.; Gong, Y.-J.; Chen, J.-L.; Goldfarb, D.; Su, X.-C. Angew. Chem., Int. Ed. 2017, 56, 2914.
Carlsson, A.-C. C.; Mehmeti, K.; Uhrbom, M.; Karim, A.; Bedin, M.; Puttreddy, R.; Kleinmaier, R.; Neverov, A. A.; Nekoueishahraki, B.; Gräfenstein, J.; Rissanen, K.; Erdélyi, M. J. Am. Chem. Soc. 2016, 138, 9853.
Heintz, K.; Imhof, W.; Görls, H. Monatsh. Chem. 2017, 148, 991.
Wan, Z.; Fang, Z.; Yang, Z.; Liu, C.; Gu, J.; Guo, K. J. Chem. Res. 2015, 39, 209.
Gilbile, R.; Bhavani, R.; Vyas, R. Orient. J. Chem. 2017, 33, 930.
Zhou, W.; Miura, T.; Murakami, M. Angew. Chem., Int. Ed. 2018, 57, 5139.
Xu, J.; Wu, W.; Wu, J. Org. Lett. 2019, 21, 5321.
Markham, J. P.; Wang, B.; Stevens, E. D.; Burris, S. C.; Deng, Y. Chem.–Eur. J. 2019, 25, 6638.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(7), 814–823
Rights and permissions
About this article
Cite this article
Baykov, S.V., Boyarskiy, V.P. Metal-Free Functionalization of Azine N-Oxides with Electrophilic Reagents. Chem Heterocycl Comp 56, 814–823 (2020). https://doi.org/10.1007/s10593-020-02737-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02737-x