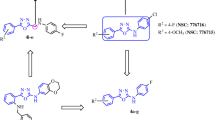
Aliphatic alkyl chain-containing 12–14-membered macrocycles have been designed as structural analogs of antimitotic natural product diazonamide A. Macrocycles were synthesized from 5-bromooxazole in 7 to 9 linear steps using Ru-catalyzed ring-closing metathesis as the key transformation. Heat effect of binding to α,β-tubulin tetramer (T4-RB3 complex) has been measured for the synthesized macrocycles by isothermal titration calorimetry method.







Similar content being viewed by others
References
van Vuuren, R. J.; Visagie, M. H.; Theron, A. E.; Joubert, A. M. Cancer Chemother. Pharmacol.2015, 76, 1101.
Cruz-Monserrate, Z.; Vervoort, H. C.; Bai, R.; Newman, D. J.; Howell, S. B.; Los, G.; Mullaney, J. T.; Williams, M. D.; Pettit, G. R.; Fenical, W.; Hamel, E. Mol. Pharmacol.2003, 63, 1273.
Wieczorek, M.; Tcherkezian, J.; Bernier, C.; Prota, A. E.; Chaaban, S.; Rolland, Y.; Godbout, C.; Hancock, M. A.; Arezzo, J. C.; Ocal, O.; Rocha, C.; Olieric, N.; Hall, A.; Ding, H.; Bramoulle, A.; Annis, M. G.; Zogopoulos, G.; Harran, P. G.; Wilkie, T. M.; Brekken, R. A.; Siegel, P. M.; Steinmetz, M. O.; Shore, G. C.; Brouhard, G. J.; Roulston, A. Sci. Transl. Med.2016, 8(365), 365ra159.
Kazak, M.; Vasilevska, A.; Suna, E. Chem. Heterocycl. Compd.2019, 56, 355. [Khim. Getrotsikl. Soedin.2019, 56, 355.]
Jeong, S.; Chen, X.; Harran, P. G. J. Org. Chem.1998, 63, 8640.
Prasad Atmuri, N. D.; Lubell, W. D. J. Org. Chem.2015, 80, 4904.
Mangold, S. L.; Grubbs, R. H. Chem. Sci.2015, 6, 4561.
Kaul, R.; Surprenant, S.; Lubell, W. D. J. Org. Chem.2005, 70, 4901.
Shibata, K.; Yoshida, M.; Doi, T.; Takahashi, T. Tetrahedron Lett.2010, 51, 1674.
Topolovčan, N.; Panov, I.; Kotora, M. Org. Lett.2016, 18, 3634.
Fürstner, A.; Guth, O.; Dueffels, A.; Seidel, G.; Liebl, M.; Gabor, B.; Mynott, R. J. Chem.–Eur. J.2001, 7, 4811.
Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett.1999, 1, 953.
Grela, K.; Harutyunyan, S.; Michrowska, A. Angew. Chem., Int. Ed.2002, 41, 4038.
Gradillas, A.; Pérez-Castells, J. Angew. Chem., Int. Ed.2006, 45, 6086.
Ojima, I.; Lin, S.; Inoue, T.; Miller, M. L.; Borella, C. P.; Geng, X.; Walsh, J. J. J. Am. Chem. Soc.2000, 122, 5343.
Muthusamy, S.; Azhagan, D. Eur. J. Org. Chem.2014, 363.
Barrett, A. G. M.; Hamprecht, D.; Ohkubo, M. J. Org. Chem.1997, 62, 9376.
Banister, S. D.; Moir, M.; Stuart, J.; Kevin, R. C.; Wood, K. E.; Longworth, M.; Wilkinson, S. M.; Beinat, C.; Buchanan, A. S.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. ACS Chem. Neurosci.2015, 6, 1546.
Acknowledgement
This work was funded by ERDF (project No. 1.1.1.1/16/A/281 “Simplified Analogues of Diazonamide A as Anticancer Agents”).
We thank Prof. K. Jaudzems for assistance with NMR experiments and the ITC assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitkovska, V., Zogota, R., Kalnins, T. et al. Aliphatic chain-containing macrocycles as diazonamide A analogs. Chem Heterocycl Comp 56, 586–602 (2020). https://doi.org/10.1007/s10593-020-02704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02704-6