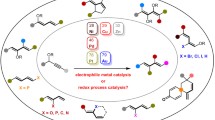
A series of 2,2-disubstituted 8-azachromanones, including spirocyclic compounds, have been synthesized via Horner–Wadsworth– Emmons reaction. Dimethyl methylphosphonate was acylated with methyl 2-methoxypyridine-3-carboxylate to afford the key intermediate – dimethyl [2-(2-methoxypyridin-3-yl)-2-oxoethyl]phosphonate. Further reaction of this phosphonate and ketones followed by treatment with TMSCl–NaI provided the target 8-azachromanones. Scope and limitations of the developed synthetic method have been investigated.

Similar content being viewed by others
References
(a) Malets, Y. S.; Moskvina, V. S.; Grygorenko, O. O.; Brovarets, V. S. Chem. Heterocycl. Compd.2019, 55, 1007. [Khim. Geterotsikl. Soedin.2019, 55, 1007.] (b) Emami, S.; Ghanbarimasir, Z. Eur. J. Med. Chem.2015, 93, 539. (c)Durham, T. B.; Blanco, M.-J. Bioorg. Med. Chem. Lett.2015, 25, 998.
(a) Nohara, A.; Ishiguro, T.; Ukawa, K.; Sugihara, H.; Maki, Y.; Sanno, Y. J. Med. Chem.1985, 28, 559. (b) Vora, K. R.; Khandwala, A.; Smith, C. G. US Patent 5362737.
(a) Khandwala, A.; Van Inwegen, R. G.; Alfano, M. C. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod.1997, 83, 222. (b) Bailey, J.; McCarthy, C.; Smith, R. F. J. Fam. Practice2011, 60, 621. (c) Burgess, J. A.; Van der Ven, P. F.; Martin, M.; Sherman, J.; Haley, J. J. Contemp. Dent. Pract.2008, 9, 88. (d) Bell, J. Clin. Drug. Invest.2005, 25, 555.
Oral, E. A.; Reilly, S. M.; Gomez, A. V.; Meral, R.; Butz, L.; Ajluni, N.; Chenevert, T. L.; Korytnaya, E.; Neidert, A. H.; Hench, R.; Rus, D.; Horowitz, J. F.; Poirier, B.; Zhao, P.; Lehmann, K.; Jain, M.; Yu, R.; Liddle, C.; Ahmadian, M.; Downes, M.; Evans, R. M.; Saltiel, A. R. Cell Metab.2017, 26, 157.
(a) Beyett, T. S.; Gan, X.; Reilly, S. M.; Gomez, A. V.; Chang, L.; Tesmer, J. J. G.; Saltiel, A. R.; Showalter, H. D. Bioorg. Med. Chem.2018, 26, 5443. (b) Gan, X.; Wilson, M. W.; Beyett, T. S.; Wen, B.; Sun, D.; Larsen, S. D.; Tesmer, J. J. G.; Saltiel, A. R.; Showalter, H. D. J. Labelled Compd. Radiopharm.2019, 62, 202. (c) Beyett, T. S.; Gan, X.; Reilly, S. M.; Chang, L.; Gomez, A. V.; Saltiel, A. R.; Showalter, H. D.; Tesmer, J. J. G. Mol. Pharmacol.2018, 94, 1210.
Okada, M.; Tokumitsu, H.; Kubota, Y.; Kobayashi, R. Biochem. Biophys. Res. Commun.2002, 292, 1023.
Brötz-Oesterhelt, H.; Knezevic, I.; Bartel, S.; Lampe, T.; Warnecke-Eberz, U.; Ziegelbauer, K.; Häbich, D.; Labischinski, H. J. Biol. Chem.2003, 278, 39435.
(a) Pankiewicz, K. W.; Chen, L.; Petrelli, R.; Felczak, K.; Gao, G.; Bonnac, L.; Yu, J. S.; Bennett, E. M. Curr. Med. Chem.2008, 15, 650. (b) Dwivedi, N.; Dube, D.; Pandey, J.; Singh, B.; Kukshal, V.; Ramachandran, R.; Tripathi, R. P. Med. Res. Rev.2008, 28, 545.
Varnes, J. G.; Marcus, A. P.; Mauger, R. C.; Throner, S. R.; Hoesch, V.; King, M. M.; Wang, X.; Sygowski, L. A.; Spear, N.; Gadient, R.; Brown, D. G.; Campbell, J. B. Bioorg. Med. Chem. Lett.2011, 21, 1402.
(a) Conn, J. P.; Tamminga, C.; Schoepp, D. D.; Lindsley, C. Mol. Interventions2008, 8, 99. (b) Lindsley, C. W.; Shipe, W. D.; Wolkenberg, S. E.; Theberge, C. R.; Williams, D. L., Jr.; Sur, C.; Kinney, G. G. Curr. Top. Med. Chem.2006, 6, 771. (c) Lindsley, C. W.; Emmitte, K. A. Curr. Opin. Drug Discovery Devel.2009, 12, 446.
(a) Madsen-Duggan, C. B.; Debenham, J. S.; Walsh, T. F.; Yan, L.; Huo, P.; Wang, J.; Tong, X.; Lao, J.; Fong, T. M.; Xiao, J. C.; Huang, C. R.; Shen, C.-P.; Stribling, D. S.; Shearman, L. P.; Strack, A. M.; Goulet, M. T.; Hale, J. J. Bioorg. Med. Chem. Lett.2010, 20, 3750. (b) Lang, R. W.; Wenk, P. F. Helv. Chim. Acta1988, 71, 596.
Yan, L.; Huo, P.; Debenham, J. S.; Madsen-Duggan, C. B.; Lao, J.; Chen, R. Z.; Xiao, J. C. Shen, C.-P.; Stribling, D. S.; Shearman, L. P.; Strack, A. M.; Tsou, N.; Ball, R. G.; Wang, J.; Tong, X.; Bateman, T. J.; Reddy, V. B. G.; Fong, T. M.; Hale, J. J. J. Med. Chem.2010, 53, 4028.
(a) Muthukaman, N.; Tambe, M.; Shaikh, M.; Pisal, D.; Deshmukh, S.; Tondlekar, S.; Sarode, N.; Narayana, L.; Gajera, J. M.; Kattige, V. G.; Honnegowda, S.; Karande, V.; Kulkarni, A.; Behera, D.; Jadhav, S. B.; Gudi, G. S.; Khairatkar-Joshi, N.; Gharat, L. A. Bioorg. Med. Chem. Lett.2017, 27, 2594. (b) Cotterill, D. W.; Johnston, D. A.; Livingstone, R. J. Chem. Res. (M)1995, 1, 221.
Cotterill, W. D.; Johnston, D. A.; Livingstone, R. J. Chem. Res. (M)1995, 6, 1466.
(a) Wadsworth, W. S., Jr.; Emmons, W. D. J. Am. Chem. Soc.1961, 83, 1733. (b) Horner, L.; Hoffmann, H.; Wippel, H. G.; Klahre, G. Chem. Ber.1959, 92, 2499. (c) Boutagy, J.; Thomas, R. Chem. Rev.1974, 74, 87. (d) Maryanoff, B. E.; Reitz, A. B. Chem. Rev.1989, 89, 863. (e) Kelly, S. E. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon: Oxford, 1991, Vol. 1, p. 729.
Somu, R. V.; Boshoff, H.; Qiao, C.; Bennett, E. M.; Barry, C. E.; Aldrich, C. C. J. Med. Chem.2006, 49, 31.
Palacios, F.; Ochoa de Retana, A. M.; Alonso, J. M. J. Org. Chem.2006, 71, 6141.
(a) Westermann, J.; Schneider, M.; Platzek, J.; Petrov, O. Org. Process Res. Dev.2007, 11, 200. (b) Paterson, I.; Lyothier, I. Org. Lett.2004, 6, 4933. (c) Milburn, R. R.; McRae, K.; Chan, J.; Tedrow, J.; Larsen, R.; Faul, M. Tetrahedron Lett.2009, 50, 870.
Eastwood, P. R.; Gonzalez Rodriguez, J.; Gomez Castillo, E.; Bach Tana, J. WO Patent 2011157397.
Grygorenko, O. O.; Volochnyuk, D. M.; Ryabukhin, S. V.; Judd, D. B. Chem.–Eur. J.2019, 25, 1.
Armarego, W. L. F.; Chai, C. L. L. Purification of Laboratory Chemicals; Elsevier: Oxford, 2003.
Acknowledgement
The work was funded by Enamine Ltd. O. O. Grygorenko and A. V. Dobrydnev received additional funding from Ministry of Education and Science of Ukraine (grant 19BF037-03).
The authors thank Prof. A. A. Tolmachev for his encouragement and support, Mr. D. V. Bylina for HRMS measurements, and reviewer for his help with elucidation of the structure of compound 11.
Electronic supplementary material
ESM 1
(PDF 2249 kb)
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(2), 213–218
Rights and permissions
About this article
Cite this article
Omelian, T.V., Ostapchuk, E.N., Dobrydnev, A.V. et al. Strategy for the synthesis of 2,2-disubstituted 8-azachromanones via Horner–Wadsworth–Emmons olefination. Chem Heterocycl Comp 56, 213–218 (2020). https://doi.org/10.1007/s10593-020-02646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02646-z