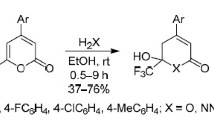
2-Cyano-4-pyrone obtained from comanic acid ethyl ester reacts with amines and hydrazines with the opening of the pyrone ring and substitution of the cyano group to form carbamoylated aminoenones (yields 62–87%) and pyrazolylacetic acid hydrazides. The reactions of 2-cyano-4-pyrone with hydroxylamine or sodium azide involve exclusively the cyano group, which makes it possible to obtain 2-heteroaryl-4-pyrones and 2-heteroaryl-4-hydroxypyridines.
Similar content being viewed by others
References
(a) Trobe, M.; Burke, M. D. Angew. Chem., Int. Ed.2018, 57, 4192. (b) Lehmann, J. W.; Blair, D. J.; Burke, M. D. Nat. Rev. Chem.2018, 2, 0115.
(a) Ghosh, C. K.; Chakraborty, A. ARKIVOC2015, (vi), 417. (b) Gao, X.; Xia, M.; Yuan, C.; Zhou, L.; Sun, W.; Li, C.; Wu, B.; Zhu, D.; Zhang, C.; Zheng, B.; Wang, D.; Guo, H. ACS Catal.2019, 9, 1645. (c) Liu, K.; Teng, H.-L.; Wang, C.-J. Org. Lett.2014, 16, 4508.
(a) Politanskaya, L. V.; Selivanova, G. A.; Panteleeva, E. V.; Tretyakov, E. V.; Platonov, V. E.; Nikulshin, P. V.; Vinogradov, A. S.; Zonov, Ya. V.; Karpov, V. M.; Mezhenkova, T. V.; Vasilyev, A. V.; Koldobskii, A. B.; Shilova, O. S.; Morozova, S. M.; Burgart, Ya. V.; Shchegolkov, E. V.; Saloutin, V. I.; Sokolov, V. B.; Aksinenko, A. Yu.; Nenajdenko, V. G.; Moskalik, M. Yu.; Astakhova, V. V.; Shainyan, B. A.; Tabolin, A. A.; Ioffe, S. L.; Muzalevskiy, V. M.; Balenkova, E. S.; Shastin, A. V.; Tyutyunov, A. A.; Boiko, V. E.; Igumnov, S. M.; Dilman, A. D.; Adonin, N. Yu.; Bardin, V. V.; Masoud, S. M.; Vorobyeva, D. V.; Osipov, S. N.; Nosova, E. V.; Lipunova, G. N.; Charushin, V. N.; Prima, D. O.; Makarov, A. G.; Zibarev, A. V.; Trofimov, B. A.; Sobenina, L. N.; Belyaeva, K. V.; Sosnovskikh, V. Ya.; Obydennov, D. L.; Usachev, S. A. Russ. Chem. Rev.2019, 88, 425. [Usp. Khim.2019, 88, 425.] (b) Usachev, B. I. J. Fluorine Chem.2015, 172, 80. b Młochowski, J.; Giurg, M.; Uher, M.; Korenova, A.; Vegh, D. J. Prakt. Chem.1996, 338, 65. c Poulton, G. A.; Williams, M. E. J. Heterocycl. Chem.1975, 12, 219. d Masanobu, I.; Atsuko, N.; Hideo, E.; Shosuke, Y. Chem. Lett.1980, 9, 1323. e Huynh-Dinh, T.; Gouyette, C.; Igolen, J. Tetrahedron Lett. 1980, 21, 4499.
(a) Honma, Y.; Sekine, Y.; Hashiyama, T.; Takeda, M.; Ono, Y.; Tsuzurahara, K. Chem. Pharm. Bull.1982, 30, 4314. (b) Shahrisa, A.; Hemmati, S. Indian J. Chem. 2000, 39B, 190.
Usachev, B. I.; Obydennov, D. L.; Röschenthaler, G.-V.; Sosnovskikh, V. Ya. J. Fluorine Chem.2012, 137, 22.
Obydennov, D. L.; Sidorova, E. S.; Usachev, B. I.; Sosnovskikh, V. Ya. Tetrahedron Lett.2013, 54, 3085.
(a) Zhou, J.; Wang, D.; Luo, X. H.; Jia, X.; Li, M. X.; Laudon, M.; Zhang, R. X.; Jia, Z. P. J. Pharmacol. Exp. Ther.2018, 364, 55. (b) Glenn, M. P.; Kahnberg, P.; Boyle, G. M.; Hansford, K. A.; Hans, D.; Martyn, A. C.; Parsons, P. G.; Fairlie, D. P. J. Med. Chem.2004, 47, 2984.
(a) Obydennov, D. L.; El-Tantawy, A. I.; Kornev, M. Yu.; Sosnovskikh, V. Ya. Mendeleev Commun. 2019, 29, 234. (b) Obydennov, D. L.; El-Tantawy, A. I.; Sosnovskikh, V. Ya. New J. Chem.2018, 42, 8943.
Obydennov, D. L.; El-Tantawy, A. I.; Sosnovskikh, V. Ya. J. Org. Chem.2018, 83, 13776.
Obydennov, D. L.; Usachev, B. I.; Sosnovskikh, V. Ya. Chem. Heterocycl. Compd.2015, 50, 1388. [Khim. Geterotsikl. Soedin.2014, 1510.]
Lu, C.-W.; Wang, Y.; Chi, Y. Chem.–Eur. J.2016, 22, 17892.
Attenburrow, J.; Elks, J.; Elliott, D. F.; Hems, B. A.; Harris, J. O.; Brodrick, C. I. J. Chem. Soc.1945, 571.
Smolyar, N. N.; Yutilov, Yu. M. Russ. J. Org. Chem.2008, 44, 1205. [Zh. Org. Khim.2008, 44, 1218.]
This work was supported by the Russian Science Foundation (grant 18-13-00186).
Elemental analysis was performed and 1H, 13C NMR spectra were recorded on equipment of the Center for collective use “Spectroscopy and analysis of organic compounds” of the Institute of Organic Synthesis, Ural Branch of the Russian Academy of Sciences and “Laboratory of comprehensive examination and expert evaluation of organic materials” of Ural Federal University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(2), 173–179
Rights and permissions
About this article
Cite this article
Obydennov, D.L., Suslova, A.I. & Sosnovskikh, V.Y. Synthesis and some chemical properties of 2-cyano-4-pyrone. Chem Heterocycl Comp 56, 173–179 (2020). https://doi.org/10.1007/s10593-020-02642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02642-3