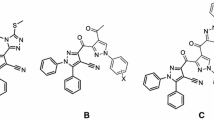
A number of azolo- and azinopyridines with varying substituents and annulated heterocycles were synthesized and examined in dearomatization reactions with carbon nucleophiles. Depending on the structure, the resulting covalent σ-adducts were formed either under basefree conditions or in Et3N-promoted process to give functionalized condensed dihydropyridines. Quantum-chemical calculations of the global electrophilicity index derived from FMO energies of azolopyridine series were performed to explain reactivity toward neutral and anionic C-nucleophiles. These values may be useful for qualitative prediction of particular reactivity pattern.




Similar content being viewed by others
References
(a) Terrier, F. Chem. Rev. 1982, 82, 77. (b) Buncel, E.; Dust, J. M.; Terrier, F. Chem. Rev. 1995, 95, 2261. (c) Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley-VCH Verlag GmbH, 2013. (d) Terrier, F. Nucleophilic Aromatic Displacement. The Influence of the Nitro Group; VCH Publishers: New York, 1991. (e) Artamkina, G. A.; Egorov, M. P.; Beletskaya, I. P. Chem. Rev.1982, 82, 427. (f) Buncel, E.; Crampton, M. R.; Strauss, M. J.; Terrier, F. Electron-Deficient Aromatic- and Heteroaromatic-Base Interactions: the Chemistry of Anion Sigma Complexes; Elsevier, 1984.
(a) Lakhdar, S.; Goumont, R.; Boubaker, T.; Mokhtari, M.; Terrier, F. Org. Biomol. Chem. 2006, 4, 1910. (b) Halle, J. C.; Pouet, M. J.; Simonin, M. P.; Terrier, F. Tetrahedron Lett. 1985, 26, 1307.
Terrier, F.; Kizilian, E.; Halle, J. C.; Buncel, E. J. Amer. Chem. Soc. 1992, 114, 1740.
(a) Terrier, F.; Simonnin, M. P.; Pouet, M. J.; Strauss, M. J. J. Org. Chem.1981, 46, 3537. (b) Terrier, F.; Millot, F.; Norris, W. P. J. Am. Chem. Soc.1976, 98, 5883.
(a) Remennikov, G. Ya.; Kempf, B.; Ofial, A. R.; Polborn, K.; Mayr, H. J. Phys. Org. Chem.2003, 16, 431. (b) Remennikov, G. Ya.; Pirozhenko, V. V.; Vdovenko, S. I.; Kravchenko, S. A. Chem. Heterocycl. Compd. 1998, 34, 104. [Khim. Geterotsikl. Soedin.1998, 112.]
Starosotnikov, A. M.; Shkaev, D. V.; Bastrakov, M. A.; Fedyanin, I. V.; Shevelev, S. A.; Dalinger, I. L. Beilstein J. Org. Chem.2017, 13, 2854.
Starosotnikov, A. M.; Shkaev, D. V.; Bastrakov, M. A.; Fedyanin, I. V.; Shevelev, S. A.; Dalinger, I. L. Mendeleev Commun.2018, 28, 638.
Lindstrom, S.; Eriksson, M.; Grivas, S. Acta Chem. Scand.1993, 47, 805.
Cikotiene, I. Eur. J. Org. Chem. 2012, 2766.
Starosotnikov, A. M.; Bastrakov, M. A.; Knyazev, D. A.; Fedyanin, I. V.; Kachala, V. V.; Dalinger, I. L. ChemistrySelect2019, 4, 1510.
Bruno, I. J.; Cole, J. C.; Kessler, M.; Luo, J.; Motherwell, W. D. S.; Purkis, L. H.; Smith, B. R.; Taylor, R.; Cooper, R. I.; Harris, S. E.; Orpen, A. G. J. Chem. Inf. Comput. Sci.2004, 44, 2133.
Fedyanin, I. V.; Lyssenko, K. A. CrystEngComm2013, 15, 10086.
Lee, S.; Diab, S.; Queval, P.; Sebban, M.; Chataigner, I.; Piettre, S. R. Chem.–Eur. J.2013, 19, 7181.
Bastrakov, M. A.; Kucherova, A. Yu.; Fedorenko, A. K.; Starosotnikov, A. M.; Fedyanin, I. V.; Dalinger, I. L.; Shevelev, S. A. ARKIVOC2017, (iii), 181.
Bastrakov, M. A.; Fedorenko, A. K.; Starosotnikov, A. M.; Kachala, V. V.; Shevelev, S. A. Chem. Heterocycl. Compd.2019, 55, 72. [Khim. Geterotsikl. Soedin.2019, 55, 72.]
(a) Vichard, D.; Halle, J.-C.; Huguet, B.; Pouet, M.-J.; Terrier, F.; Riou, D. Chem. Commun.1998, 791. (b) Sepulcri, P.; Halle, J. C.; Goumont, R.; Riou, D.; Terrier, F. J. Org. Chem.1999, 64, 9254. (c) Halle, J.-C.; Vichard, D.; Pouet, M.-J.; Terrier, F. J. Org. Chem.1997, 62, 7178. (d) Terrier, F.; Sebban, M.; Goumont, R.; Halle, J. C.; Moutiers, G.; Cangelosi, I.; Buncel, E. J. Org. Chem.2000, 65, 7391.
Parr, R. G.; Szenpaly, L. V.; Liu, S. J. Am. Chem. Soc.1999, 122, 1922.
Perez, P.; Domingo, L. R.; Aizman, A.; Contreras, R. In Theoretical Aspects of Chemical Reactivity; Toro-Labbe, A., Ed.; Elsevier Science, 2007, Chapter 9, p. 139.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A., Jr.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 03, Revision C.02; Gaussian, Inc., 2004.
Starosotnikov, A. M.; Khakimov, D. V.; Bastrakov, M. A.; Pechenkin, S. Yu.; Shevelev, S. A.; Pivina, T. S. Chem. Heterocycl. Compd.2011, 47, 215. [Khim. Geterotsikl. Soedin. 2011, 271.]
Cai, S. X.; Huang, J.-C.; Espitia, S. A.; Tran, M.; Ilyin, V. I.; Hawkinson, J. E.; Woodward, R. M.; Weber, E.; Keana, J. F. W. J. Med. Chem.1997, 40, 3679.
Wu, R.; Smidansky, E. D.; Oh, H. S.; Takhampunya, R.; Padnamabhan, R.; Cameron, C. E.; Peterson, B. R. J. Med. Chem. 2010, 53, 7958.
Edmunds, A.; Muehlebach, M.; Stoller, A.; Loiseleur, O.; Buchholz, A.; Hueter, O. F.; Bigot, A.; Hall, R. G.; Emery, D.; Jung, P. J. M.; Lu, L.; Wu, Y.; Chen R. WO Patent 2015000715.
Bhakuni, D. S.; Shoeb, A.; Popli, S. P. Indian J. Chem. 1968, 6, 123.
Boyer, J. H.; Schoen, W. J. Am. Chem. Soc.1956, 78, 423.
Cmoch, P.; Kamienski, B.; Kamienska-Trela, K.; Stefaniak, L.; Webb, J. A. J. Phys. Org. Chem. 2000, 13, 480.
Leyva, E.; de Loera, D.; Rogelio, J.-C. Tetrahedron Lett. 2010, 51, 3978.
Israel, M.; Day, A. R. J. Org. Chem. 1959, 24, 1455.
Shi, J.; Stover, J. S.; Whitby, L. R.; Vogt, P. K.; Boger, D. L. Bioorg. Med. Chem. Lett.2009, 19, 6038.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv.2015, A71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem.2015, C71, 3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(1), 92–100
Electronic supplementary material
ESM 1
(PDF 11803 kb)
This work was supported by the Russian Science Foundation (grant 19-73-20259). X-ray diffraction studies were performed with financial support from the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for molecular composition studies of the A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences.
Rights and permissions
About this article
Cite this article
Starosotnikov, A.M., Ilkov, K.V., Bastrakov, M.A. et al. Mild and efficient addition of carbon nucleophiles to condensed pyridines: influence of structure and limits of applicability. Chem Heterocycl Comp 56, 92–100 (2020). https://doi.org/10.1007/s10593-020-02628-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02628-1