
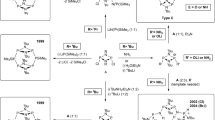
As a result of the implementation of two approaches, the Pudovik reaction (the reaction of macrocyclic azomethines with secondary phosphine oxides) and the Kabachnik–Fields reaction (three-component one-pot process involving dialdehydes, diamines, and secondary phosphine oxides), novel N,O-containing macrocyclic ligands with phosphine oxide groups were first obtained. The synthesized macrocycles are a kind of α-aminophosphoryl compounds that can be used in the synthesis of supramolecular systems.


Similar content being viewed by others
References
Park, H. S.; Lin, Q.; Hamilton, A. D. J. Am. Chem. Soc.1999, 121, 8.
Tabushi, I.; Yamamura, K. Top. Curr. Chem.1983, 113, 146.
Reyes-Marquez, V.; Tiburcio, J.; Hopfl, H.; Sanchez-Vazquez, M.; Hernandez-Ahuactzi, I. F.; Moreno-Corral, R.; Lara, K. O. J. Phys. Org. Chem. 2012, 25, 1042.
Erkin, A. V.; Gurzhiy, V. V.; Krutikov, V. I. Chem. Heterocycl. Compd.2018, 54, 1168. [Khim. Geterotsikl. Soedin.2018, 54, 1168.]
Ostrovskii, V. A.; Mazur, M. S.; Mikhailenko, V. V.; Aksenov, N. A.; Aksenov, A. V. Chem. Heterocycl. Compd.2016, 52, 849. [Khim. Geterotsikl. Soedin.2016, 52, 849.]
Balic, T.; Markovic, B.; Jazwinski, J.; Matkovic-Calogovic, D. Inorg. Chim. Acta2015, 435, 283.
Vicente, M.; Bastida, R.; Lodeiro, C.; Macias, A.; Parola, A. J.; Valencia, L.; Spey, S. E. Inorg. Chem.2003, 42, 6768.
Rohovec, J.; Vojtisek, P.; Lukes, I. Phosphorus, Sulfur Silicon Relat. Elem.1999, 148, 79.
Bilge, S.; Nadzagdorj, A.; Akduran, N.; Hokelek, T.; Kilic, Z. J. Mol. Struct.2002, 611, 169.
Ilter, E. E.; Kilic, Z. Spectrochim. Acta, Part A2008, 70, 542.
Bilge, S.; Kilic, Z.; Davies, D. B. Spectrochim. Acta, Part A2011, 81, 441.
Kocak, S. B.; Uckardes, H. Heteroat. Chem.2012,23, 583.
Trishin, Yu. G.; Kudryavtseva, A. I.; Shafeeva, M. V.; Avdeeva, E. A.; Karpova, E. A. Russ. J. Gen. Chem.2013, 83, 2345. [Zh. Obshch. Khim.2013, 83, 2062.]
Cherkasov, R. A.; Galkin, V. I. Russ. Chem. Rev.1998, 67, 857. [Usp. Khim.1998, 67, 940.]
Stid, Dzh. V.; Etvud, Dzh. L. Supramolecular Chemistry [Russian translation]; IKTs "Akademkniga": Moscow, 2007, Vol. 1, p. 159.
Kedy, S.; Almhna, N.; Kandil, F. Jordan J. Chem.2012, 7, 73.
Beider, R. Atoms in Molecules. A Quantum Theory [Russian translation]; Mir: Moscow, 2000.
Moreno-Corral, R.; Hopfl, H.; Machi-Lara, L.; Lara, K. O. Eur. J. Org. Chem.2011, 2148.
Gel'man, N. E.; Terent’eva, E. A; Shanina, T. M.; Kiparenko, L. М.; Rezl, V. Methods of Quantitative Organic Elemental Microanalysis [in Russian]; Gel'man, N. E., Ed.; Khimiya: Moscow, 1987.
Keglevich, G.; Jablonkai, E.; Balazs, L. B. RSC Adv.2014, 4, 22808.
Kabat, M. M.; Garofalo, L. M.; Daniewski, A. R.; Hutchings, S. D; Liu, W.; Okabe, M.; Radinov, R.; Zhou, Y. J. Org. Chem.2001, 66, 6141.
Yapar, G.; Erk, C. J. Inclusion Phenom. Macrocyclic Chem.2002, 43, 299.
Adam, K. R.; Leong, A. J.; Lindoy, L. F.; Lip, H. C.; Skelton, B. W.; White, A. H. J. Am. Chem. Soc.1983, 105, 4645.
Sheldrick, G. M. SHELXTL v.6.14. Structure Determination Software Suite; Bruker AXS: Madison, 2000.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, 2016.
Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. J. Chem. Phys.2010, 132, 154104.
Grimme, S.; Ehrlich, S.; Goerigk, L. J. Comput. Chem.2011, 32, 1456.
Keith, T. AIMAll (Version 16.08.17); TK Gristmill Software: Overland Park, 2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(9), 875–881
Rights and permissions
About this article
Cite this article
Alexandrova, E.А., Lotsman, K.А., Lyssenko, K.А. et al. Synthesis of novel N,O-macrocyclic ligands, functionalized by phosphine oxide groups. Chem Heterocycl Comp 55, 875–881 (2019). https://doi.org/10.1007/s10593-019-02551-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02551-0


