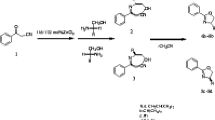
A simple and convenient approach to the synthesis of N-substituted 1,3-oxazolidines via a hetero-domino Petasis borono-Mannich reaction of 1,2-amino alcohols, formaldehyde, and organoboronic acids has been reported. The transformation provides an effective and complementary pathway toward 1,3-oxazolidine derivatives.
Similar content being viewed by others
References
Wolf, C.; Xu, H. Chem. Commun. 2011, 47, 3339.
Nakano, H.; Okuyama, Y.; Kwon, E. Heterocycles 2014, 89, 1.
Gazaliev, A. M.; Nurkenov, O. A.; Kabieva, S. K.; Dauletzhanova, Z. T.; Seilkhanov, T. M.; Fazylov, S. D.; Ibraev, M. K.; Isaeva, A. Z. Chem. Nat. Compd. 2017, 53, 1005. [Khim. Prir. Soedin. 2017, 853.]
Ceylan, S.; Bayrak, H.; Ozdemir, S. B.; Uygun, Y.; Mermer, A.; Demirbas, N.; Ulker, S. Lett. Org. Chem. 2016, 13, 636.
Basoglu, S.; Ulker, S.; Alpay-Karaoglu, S.; Demirbas, N. Med. Chem. Res. 2014, 23, 3128.
Hahn, H.-G.; Oh, H. S.; Cheon, S. H.; Oak, M. H.; Kim, Y.-R.; Kim, K.-M. Arch. Pharm. Res. 2004, 27, 518.
Gonçalves, R. S. B.; Kaiser, C. R.; Lourenço, M. C. S.; de Souza, M. V. N.; Wardell, J. L.; Wardell, S. M. S. V.; da Silva, A. D. Eur. J. Med. Chem. 2010, 45, 6095.
Hirai, K.; Yano, T.; Matsukawa, T.; Ugai, S.; Nagato, S.; Hori, M. J. Pestic. Sci. 1999, 24, 156.
(a) Kuhnert, N.; Danks, T. N. Green Chem. 2001, 3, 68. (b) Khabibullina, G. P.; Yanybin, V. M.; Ibragimov, A. G.; Dzhemilev, U. M. Chem. Heterocycl. Compd. 2014, 50, 726. [Khim. Geterotsikl. Soedin. 2014, 788.] (c) Fu, Y.; Chen, W.-G.; Hou, Y.-W.; Wang, B.; Zhao, L.-X.; Ye, F. J. Heterocycl. Chem. 2017, 54, 1660.
Meyer, A. G.; Ryan, J. H. Molecules 2016, 21, 935.
Cardoso, A. L.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2012, 6479.
Létinois, S.; Dumur, J.-C.; Hénin, F.; Muzart, J. Tetrahedron Lett. 1998, 39, 2327.
Nishitani, T.; Shiraishi, H.; Sakaguchi, S.; Ishii, Y. Tetrahedron Lett. 2000, 41, 3389.
Michaelis, D. J.; Shaffer, C. J.; Yoon, T. P. J. Am. Chem. Soc. 2007, 129, 1866.
Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2010, 132, 4570.
Nimmagadda, S. K.; Zhang, Z.; Antilla, J. C. Org. Lett. 2014, 16, 4098.
Hou, Y.; Qin, M.; Yang, X.; Shen, Q.; Zhao, Y.; Liu, Y.; Gong, P. RSC Adv. 2017, 7, 7401.
(a) Zhao, Y.; Sun, L.; Zeng, T.; Wang, J.; Peng, Y.; Song, G. Org. Biomol. Chem. 2014, 12, 3493. (b) Wang, J.; Shen, Q.; Li, P.; Peng, Y.; Song, G. Org. Biomol. Chem. 2014, 12, 5597. (c) Wang, J.; Li, P.; Shen, Q.; Song, G. Tetrahedron Lett. 2014, 55, 3888. (d) Wang, J.; Shen, Q.; Zhang, J.; Song, G. Tetrahedron. Lett. 2015, 56, 903. (e) Zhang, J.-Y.; Huang, X.; Shen, Q.-Y.; Wang, J.-Y.; Song, G.-H. Chin. Chem. Lett. 2018, 29, 197. (f) Sun, L.; Wu, M.; Huang, X.; Wang, J.; Song, G. Chem. Heterocycl. Compd. 2018, 54, 355. [Khim. Geterotsikl. Soedin. 2018, 54, 355.]
Heaney, H.; Papageorgiou, G.; Wilkins, R. F. Tetrahedron 1997, 53, 14381.
Weinberg, N. L.; Brown, E. A. J. Org. Chem. 1966, 31, 4054.
Ryan, J. H.; Spiccia, N.; Wong, L. S.-M.; Holmes, A. B. Aust. J. Chem. 2007, 60, 898.
Financial support for this work from the National Key R&D Program (grant No. 2017YFD0200504), the National Natural Science Foundation of China (grant No. 21572060), the Shanghai Key Laboratory of Catalysis Technology for Polyolefins (LCTP-201301), and the Fundamental Research Funds for the Central Universities is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing 1H and 13C NMR spectral data of compounds 4a–r, 4t, and 4u, is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(7), 648–653
Electronic supplementary material
ESM 1
(PDF 3744 kb)
Rights and permissions
About this article
Cite this article
Zheng, Y., Sun, L., Wang, J. et al. Direct synthesis of N-substituted 1,3-oxazolidines via a hetero-domino Petasis borono-Mannich reaction of 1,2-amino alcohols, formaldehyde, and organoboronic acids. Chem Heterocycl Comp 55, 648–653 (2019). https://doi.org/10.1007/s10593-019-02511-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02511-8