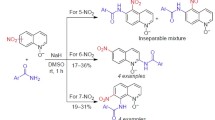
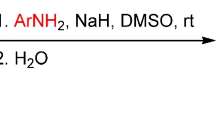
Direct SNH amidation of 5-, 6-, 7-, and 8-nitroquinolines in anhydrous DMSO was used to obtain the respective aroylamino derivatives of nitro- and nitrosoquinolines.



Similar content being viewed by others
References
(a) Michael, J. P. Nat. Prod. Rep. 1997, 14, 605. (b) Kumar, S.; Bawa, S.; Gupta, H. Mini-Rev. Med. Chem. 2009, 9, 1648. (c) Puskullu, M. O.; Tekiner, B.; Suzen, S. Mini-Rev. Med. Chem. 2013, 13, 365. (d) Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845. (e) Gopaul, K.; Shintre, S. A.; Koorbanally, N. A. Anticancer Agents Med. Chem. 2015, 15, 631. (f) Hussaini, S. M. Expert Opin. Ther. Pat. 2016, 26, 1201. (g) Jain, S.; Chandra, V.; Jain, P. K.; Pathak, K.; Pathak, D.; Vaidya, A. Arab. J. Chem. 2016. DOI:https://doi.org/10.1016/j.arabjc.2016.10.009. (h) Sharma, V.; Mehta, D. K.; Das, R. Mini-Rev. Med. Chem. 2017, 17, 1557. (i) Musiol, R. Expert Opin. Drug Discovery 2017, 12, 583.
Mąkosza, M.; Wojciechowski, K. Top. Heterocycl. Chem. 2014, 37, 51.
(a) Baeten, M.; Maes, B. U.W. Adv. Organomet. Chem. 2017, 67, 401. (b) C–H Bond Activation and Catalytic Functionalization. I and II; Dixneuf, P. H.; Doucet, H., Eds.; Springer: Berlin, 2016. (c) Metal Free C–H Functionalization of Aromatics. Nucleophilic Displacement of Hydrogen; Charushin, V.; Chupakhin, O., Eds.; Springer: Cham, 2014.
(a) Arends, I.; Sheldon, R.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, 2007. (b) Constable, D. J. C.; Dunn, P. J.; Hayler, J. D.; Humphrey, G. R.; Leazer, J. L.; Linderman, R. J.; Lorenz, K.; Manley, J.; Pearlman, B. A.; Wells, A.; Zaks, A.; Zhang, T. Y. Green Chem. 2007, 9, 411. (c) Utepova, I. A.; Trestsova, M. A.; Chupakhin, O. N.; Charushin, V. N.; Rempel, A. A. Green Chem. 2015, 17, 4401. (d) Lancaster, M. Green Chemistry. An Introductory Text; 2nd ed.; RSC Publishing: Cambridge, 2010. (e) Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437.
(a) Kim, J.; Kim, J.; Chang, S. Chem.–Eur. J. 2013, 19, 7328. (b) Ryu, J.; Shin, K.; Park, S. H.; Kim, J. Y.; Chang, S. Angew. Chem. 2012, 124, 10042. (c) Shi, J.; Zhou, B.; Yang, Y.; Li, Y. Org. Biomol. Chem. 2012, 10, 8953.
(a) Verbitskiy, E. V.; Cheprakova, E. M.; Slepukhin, P. A.; Kravchenko, M. A.; Skornyakov, S. N.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Eur. J. Med. Chem. 2015, 97, 225. (b) Verbitskiy, E. V.; Cheprakova, E. M.; Subbotina, J. O.; Schepochkin, A. V.; Slepukhin, P. A.; Rusinov, G. L.; Charushin, V. N.; Chupakhin, O. N.; Makarova, N. I.; Metelitsa, A. V.; Minkin, V. I. Dyes Pigm. 2014, 100, 201.
(a) Chupakhin, O. N.; Charushin, V. N.; van der Plas, H. C. Nucleophilic Aromatic Substitution of Hydrogen; Academic Press: San Diego, 1994. (b) Chupakhin, O. N.; Charushin, V. N. Tetrahedron Lett. 2016, 57, 2665. (c) Charushin, V. N.; Chupakhin, O. N. Top. Heterocycl. Chem. 2014, 37, 1. (d) Gulevskaya, A. V.; Pozharskii, A. F. Top. Heterocycl. Chem. 2014, 37, 179. (e) Mąkosza, M. Synthesis 2017, 3247. (f) Suwiński, J. W. ARKIVOC 2017, (i), 402. (g) Czaban-Jóźwiak, J.; Loska, R.; Mąkosza, M. J. Org. Chem. 2016, 81, 11751. (h) Varaksin, M. V.; Utepova, I. A.; Chupakhin, O. N. Chem. Heterocycl. Compd. 2012, 48, 1213. [Khim. Geterotsikl. Soedin. 2012, 1301.] (i) Varaksin, M. V.; Utepova, I. A.; Chupakhin, O. N.; Charushin, V. N. Tetrahedron 2015, 71, 7077.
(a) Matern, A. I.; Charushin, V. N.; Chupakhin, O. N. Russ. Chem. Rev. 2007, 76, 23. [Usp. Khim. 2007, 76, 27.] (b) Chupakhin, O. N.; Charushin, V. N.; van der Plas, H. C. Tetrahedron 1988, 44, 1. b Berberova, N. T.; Okhlobystin, O. Yu. Chem. Heterocycl. Compd. 1984, 20, 817. [Khim. Geterotsikl. Soedin. 1984, 1011.]
Budyka, M. F.; Terent'ev, P. B.; Kost, A. N. Chem. Heterocycl. Compd. 1978, 14, 663. [Khim. Geterotsikl. Soedin. 1978, 809.]
Borovlev, I. V.; Demidov, O. P.; Saigakova, N. A.; Amangasieva, G. A. Eur. J. Org. Chem. 2014, 7675.
(a) Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Steglenko, D. V.; Minkin, V. I.; Rusinov, G. L.; Matern, A. I. RSC Adv. 2016, 6, 77834. (b) Makhaeva, G. F.; Lushchekina, S. V.; Boltneva, N. P.; Serebryakova, O. G.; Rudakova, E. V.; Ustyugov, A. A.; Bachurin, S. O.; Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Richardson, R. J. Bioorg. Med. Chem. 2017, 25, 5981. (c) Shchepochkin, A. V.; Chupakhin, O. N.; Charushin, V. N.; Rusinov, G. L.; Subbotina, Yu. O.; Slepukhin, P. A.; Budnikova, Yu. G. Russ. Chem. Bull., Int. Ed. 2013, 62, 773. [Izv. Akad. Nauk, Ser. Khim. 2013, 772.]
Gulevskaya, A. V.; Tyaglivaya, I. N.; Verbeeck, S.; Maes, B. U. W.; Tkachuk, A. V. ARKIVOC 2011, (ix), 238.
Garnier, E.; Audoux, J.; Pasquinet, E.; Suzenet, F.; Poullain, D.; Lebret, B.; Guillaumet, G. J. Org. Chem. 2004, 69, 7809.
(a) Bashkin, J. K.; Rains, R.; Stern, M. Green Chem. 1999, 1, G41. (b) Triplett, R. D.; Rains, R. K. US Patent 7504539.
Patriciu, O.-I.; Fînaru, A.-L.; Săndulescu, I.; Guillaumet, G. Synthesis 2007, 3868.
(a) Tondys, H.; van der Plas, H. C.; Wozniak, M. J. Heterocycl. Chem. 1985, 22, 353. (b) Wozniak, M.; Baranski, A.; Nowak, K.; van der Plas, H. C. J. Org. Chem. 1987, 52, 5643.
Demidov, O. P.; Pobedinskaya, D. Yu.; Avakyan, E. K.; Amangasieva, G. A.; Borovlev, I. V. Chem. Heterocycl. Compd. 2018, 54, 875. [Khim. Geterotsikl. Soedin. 2018, 54, 875].
(a) Grzegożek, M. J. Heterocycl. Chem. 2008, 45, 1879. (b) Grzegożek, M.; Szpakiewicz, B.; Kowalski, P. ARKIVOC 2009, (vi), 84.
(a) Stern, M. K.; Cheng, B. K. J. Org. Chem. 1993, 58, 6883. (b) Esser, F.; Pook, K.-H. Synthesis 1992, 596.
Borovlev, I. V.; Demidov, O. P.; Kurnosova, N. A.; Amangasieva, G. A.; Avakyan, E. K. Chem. Heterocycl. Compd. 2015, 51, 170. [Khim. Geterotsikl. Soedin. 2015, 51, 170.]
Demidov, O. P.; Borovlev, I. V.; Amangasieva, G. A.; Avakyan, E. K. Chem. Heterocycl. Compd. 2016, 52, 104. [Khim. Geterotsikl. Soedin. 2016, 52, 104.]
Amangasieva, G. A.; Borovlev, I. V.; Demidov, O. P.; Avakyan, E. K.; Borovleva, A. A. Russ. J. Org. Chem. 2018, 54, 867. [Zh. Org. Khim. 2018, 54, 865.]
(a) Borovlev, I. V.; Demidov, O. P.; Amangasieva, G. A.; Avakyan, E. K.; Borovleva, A. A.; Pobedinskaya, D. Yu. Synthesis 2018, 3520. (b) Avakyan, E. K.; Borovlev, I. V.; Demidov, O. P.; Amangasieva, G. A.; Pobedinskaya, D. Yu. Chem. Heterocycl. Compd. 2017, 53, 1207. [Khim. Geterotsikl. Soedin. 2017, 53, 1207.]
(a) Wróbel, Z.; Kwast, A. Synlett 2007, 1525. (b) Wróbel, Z.; Kwast, A. Synthesis 2010, 3865. (c) Kwast, A.; Stachowska, K.; Trawczyński, A.; Wróbel, Z. Tetrahedron Lett. 2011, 52, 6484. (d) Wróbel, Z.; Stachowska, K.; Grudzień, K.; Kwast, A. Synlett 2011, 1439. (e) Wróbel, Z.; Więcław, M.; Bujok, R.; Wojciechowski, K. Monatsh. Chem. 2013, 144, 1847.
Khan, B.; Khan, A. A.; Bora, D.; Verma, D.; Koley, D. ChemistrySelect 2017, 2, 260.
Gottlieb, H. E.; Kotlyar, V.; Nudelman, A. J. Org. Chem. 1997, 62, 7512.
Sharp, J. T.; Gosney, I.; Rowley, A. G. Practical Organic Chemistry; Chapman and Hall: London, 1989.
(a) Zhu, X.; Qiao, L.; Ye, P.; Ying, B.; Xu, J.; Shen, C.; Zhang, P. RSC Adv. 2016, 6, 89979. (b) He, Y.; Zhao, N.; Qiu, L.; Zhang, X.; Fan, X. Org. Lett. 2016, 18, 6054. (c) Mondal, S.; Samanta, S.; Hajra, A. Adv. Synth. Catal. 2018, 360, 1026. (d) Whiteoak, C. J.; Planas, O.; Company, A.; Ribas, X. Adv. Synth. Catal. 2016, 358, 1679. (e) Wang, Y.; Yu, F.; Han, X.; Li, M.; Tong, Y.; Ding, J.; Hou, H. Inorg. Chem. 2017, 56, 5953.
Ma̧kosza, M.; Białecki, M. J. Org. Chem. 1998, 63, 4878.
CrysAlisPro, version 1.171.38.41; Rigaku Oxford Diffraction, 2015. https://www.rigaku.com/en/products/smc/crysalis.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(7), 623–631
Electronic supplementary material
ESM 1
(PDF 1726 kb)
Rights and permissions
About this article
Cite this article
Amangasieva, G.А., Avakyan, E.K., Demidov, O.P. et al. SNH Amidation of nitroquinolines: synthesis of amides on the basis of nitro- and nitrosoquinolines. Chem Heterocycl Comp 55, 623–631 (2019). https://doi.org/10.1007/s10593-019-02508-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02508-3