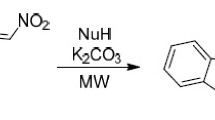
Synthetic approach toward indole derivatives bearing 2-nitroethyl group and polar azole moiety has been developed. This method involves conjugate addition of 1,3-dicarbonyl compounds to 3-(2-nitrovinyl)-1H-indoles followed by cyclocondensation with hydrazine or hydroxylamine to furnish pyrazole or isoxazole ring. Application of microwave activation allows to obtain the target indole derivatives in short time and avoid protection of indole nitrogen atom.

Similar content being viewed by others
References
(a) Stempel, E.; Gaich, T. Acc. Chem. Res. 2016, 49, 2390. (b) Speck, K.; Magauer, T. Beilstein J. Org. Chem. 2013, 9, 2048. (c) Fu, L. In Topics in Heterocyclic Chemistry; Gribble, G. W., Ed.; Springer: Heidelberg, 2010, Vol. 26, p. 433.
Aksenov, A. V.; Smirnov, A. N.; Magedov, I. V.; Reisenauer, M. R.; Aksenov, N. A.; Aksenova, I. V.; Pendleton, A. L.; Nguyen, G.; Johnston, R. K.; Rubin, M.; De Carvalho, A.; Kiss, R.; Mathieu, V.; Lefranc, F.; Correa, J.; Cavazos, D. A.; Brenner, A. J.; Bryan, B. A.; Rogelj, S.; Kornienko, A.; Frolova, L. V. J. Med. Chem. 2015, 58, 2206.
Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Aksenova, I. V.; Frolova, L. V.; Kornienko, A.; Magedov, I. V.; Rubin, M. Chem. Commun. 2013, 49, 9305.
Aksenov, A. V.; Aksenov, N. A.; Skomorokhov, A. A.; Aksenova, I. V.; Gryaznov, G. D.; Voskressensky, L. G.; Rubin, M. A. Chem. Heterocycl. Compd. 2016, 52, 923. [Khim. Geterotsikl. Soedin. 2016, 52, 923.]
(a) Kaur, J.; Islam, N.; Kumar, A.; Bhardwaj, V. K.; Chimni, S. S. Tetrahedron 2016, 72, 8042. (b) Hahn, R.; Raabe, G.; Enders, D. Org. Lett. 2014, 16, 3636.
(a) Mahboobi, S.; Grothus, G.; Meindl, W. Arch. Pharm. 1994, 327, 105. (b) Calvaire, A.; Pallaud, R. Compt. Rend. 1964, 258, 609.
(a) Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339. (b) Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786. (c) Sheldrick, G. M. Acta Crystallogr., Sect. A.: Found. Crystallogr. 2008, A64, 112.
This research was financially supported by the Grants Council of the President of the Russian Federation for state support of young Russian scientists (grant MK-3089.2018.3) and the Ministry of Education and Science of the Russian Federation (projects 4.5547.2017/8.9, 4.1196.2017/4.6, and 02.a03.21.0008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(6), 541–546
Rights and permissions
About this article
Cite this article
Aksenov, N.A., Skomorokhov, A.A., Aksenov, A.V. et al. Michael addition to 3-(2-nitrovinyl)indoles – route toward aliphatic nitro compounds with heterocyclic substituents. Chem Heterocycl Comp 55, 541–546 (2019). https://doi.org/10.1007/s10593-019-02493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02493-7