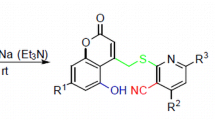
Reactions of 4Н-chromene-3-carbaldehydes and 1Н-benzo[f]chromene-3-carbaldehydes with 4-aminocoumarin were used to obtain a series of pyridocoumarin derivatives containing a 2-hydroxybenzyl or 2-hydroxy-1-naphthyl substituent at the β-position relative to the nitrogen atom. The reaction mechanism includes a Michael reaction, chromane ring opening, and cyclodehydration.

Similar content being viewed by others
References
(a) Mandal, T. K.; Kuznetsov, V. V.; Soldatenkov, A. T. Chem. Heterocycl. Compd. 1994, 30, 867. [Khim. Geterotsikl. Soedin. 1994, 1011.] (b) Núñez-Vergara, L. J.; Squella, J. A.; Navarrete-Encina, P. A.; Vicente-García, E.; Preciado, S.; Lavilla, R. Curr. Med. Chem. 2011, 18, 4761.
(a) Thapa, P.; Jun, K.-Y.; Kadayat, T. M.; Park, C.; Zheng, Z.; Magar, T. B. T.; Bist, G.; Shrestha, A.; Na, Y.; Kwon, Y.; Lee, E.-S. Bioorg. Med. Chem. 2015, 23, 6454. (b) Thapa, U.; Thapa, P.; Karki, R.; Yun, M.; Choi, J. H.; Jahng, Y.; Lee, E.; Jeon, K.-H.; Na, Y.; Ha, E.-M.; Cho, W.-J.; Kwon, Y.; Lee, E.-S. Eur. J. Med. Chem. 2011, 46, 3201. (c) Neves, M. A. C.; Dinis, T. C. P.; Colombo, G.; Sá e Melo, M. L. Eur. J. Med. Chem. 2009, 44, 4121. (d) Mulakayala, N.; Rambabu, D.; Raja, M. R.; Chaitanya, M.; Kumar, C. S.; Kalle, A. M.; Krishna, G. R.; Reddy, C. M.; Rao, M. V. B.; Pal, M. Bioorg. Med. Chem. 2012, 20, 759. (e) Thapa, P.; Lee, E.-S. Bull. Korean Chem. Soc. 2012, 33, 3103.
Hegab, M. I.; Abdel-Fattah, A.-S. M.; Yousef, N. M.; Nour, H. F.; Mostafa, A. M.; Ellithey, M. Arch. Pharm. Chem. Life Sci. 2007, 340, 396.
Dawane, B. S.; Konda, S. G.; Bodade, R. G.; Bhosale, R. B. J. Heterocycl. Chem. 2010, 47, 237.
(a) Chen, H.; Huang, M.; Li, X.; Liu, L.; Chen, B.; Wang, J.; Lin, Y. Fitoterapia 2018, 124, 103. (b) Huang, S. Z.; Cheng, B. H.; Ma, Q. Y.; Wang, Q.; Kong, F. D.; Dai, H. F.; Qiu, S. Q.; Zheng, P. Y.; Liu, Z. Q.; Zhao, Y.-X. RSC Adv. 2016, 6, 21139. (c) Zhou, F.-J.; Nian, Y.; Yan, Y.; Gong, Y.; Luo, Q.; Zhang, Y.; Hou, B.; Zuo, Z.-L.; Wang, S.-M.; Jiang, H.-H.; Yang, J.; Cheng, Y.-X. Org. Lett. 2015, 17, 3082. (d) Luo, Q.; Yang, X.-H.; Yang, Z.-L.; Tu, Z.-C.; Cheng, Y.-X. Tetrahedron 2016, 72, 4564. (e) Wang, X.-L.; Dou, M.; Luo, Q.; Cheng, L.-Z.; Yan, Y.-M.; Li, R.-T.; Cheng, Y.-X. Fitoterapia 2017, 116, 93. (f) Zhao, Z.-Z.; Chen, H.-P.; Feng, T.; Li, Z.-H.; Dong, Z.-J.; Liu, J.-K. J. Asian Nat. Prod. Res. 2015, 17, 1160.
Osipov, D. V.; Osyanin, V. A.; Klimochkin, Yu. N. Targets Heterocycl. Syst. 2018, 22, 436.
Ivanov, I. C.; Karagiosov, S. K.; Simeonov, M. F. Arch. Pharm. (Weinheim) 1991, 324, 61.
Osipov, D. V.; Osyanin, V. A.; Klimochkin, Yu. N. Chem. Heterocycl. Compd. 2018, 54, 1121. [Khim. Geterotsikl. Soedin. 2018, 54, 1121.]
(a) Ling, F.; Xiao, L.; Fang, L.; Lv, Y.; Zhong, W. Adv. Synth. Catal. 2018, 360, 444. (b) Yadav, A.; Biswas, S.; Mobin, S. N.; Samanta, S. Tetrahedron Lett. 2017, 58, 3634. (c) Adib, M.; Peytam, F.; Rahmanian-Jazi, M.; Mohammadi-Khanaposhtani, M.; Mahernia, S.; Bijanzadeh, H. R.; Jahani, M.; Imanparast, S.; Faramarzi, M. A.; Mahdavi, M.; Larijani, B. New J. Chem. 2018, 42, 17268. (d) Weng, Y.; Zhou, H.; Sun, C.; Xie, Y.; Su, W. J. Org. Chem. 2017, 82, 9047. (e) Lee, Y. R. KR Patent 20180003668A.
(a) Osipov, D. V.; Osyanin, V. A.; Klimochkin, Yu. N. Russ. Chem. Rev. 2017, 86, 625. [Usp. Khim. 2017, 86, 625.] (b) Lukashenko, A. V.; Osyanin, V. A.; Osipov, D. V.; Klimochkin, Yu. N. J. Org. Chem. 2017, 82, 1517. a Lukashenko, A. V.; Osipov, D. V.; Osyanin, V. A.; Klimochkin, Yu. N. Russ. J. Org. Chem. 2016, 52, 1817. [Zh. Org. Khim. 2016, 52, 1824.] (d) Lukashenko, A. V.; Osyanin, V. A.; Osipov, D. V.; Klimochkin, Yu. N. Chem. Heterocycl. Compd. 2016, 52, 711. [Khim. Geterotsikl. Soedin. 2016, 52, 711.]
The reported study was funded by the Russian Foundation for Basic Research and the Samara Oblast according to the research project 17-43-630838 and was also supported by the Ministry of Education and Science of the Russian Federation within the framework of State Assignment (project 4.5628.2017/6.7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(3), 261–265
Rights and permissions
About this article
Cite this article
Osipov, D.V., Artyomenko, A.A., Osyanin, V.A. et al. The reaction of 4-aminocoumarin with β-carbonyl-substituted 4Н-chromenes: synthesis of 5H-chromeno[4,3-b]pyridin-5-one derivatives. Chem Heterocycl Comp 55, 261–265 (2019). https://doi.org/10.1007/s10593-019-02451-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02451-3