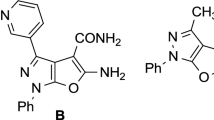
Efficient and regioselective on-water synthesis of variously substituted 5-amino-4,7-dihydropyrazolo[1,5-a]pyrimidine-6-carbonitriles and 5-amino-4,7-dihydro[1,2,4]triazolo[1,5-a]pyrimidine-6-carbonitriles using 1,8-diazabicyclo[5.4.0]undec-7-ene as catalyst has been demonstrated. The reaction of 2-benzylidenemalononitriles and 3-aryl-1H-pyrazol-5-amines or 1H-1,2,4-triazol-5-amine allows to obtain the respective pyrazolo[1,5-a]pyrimidines and [1,2,4]triazolo[1,5-a]pyrimidines in high yields up to 93%. Main advantages of the developed synthetic protocol are application of water as environmentally friendly solvent, short reaction times, simple workup procedure that often does not require further purification of products, and broad substrate scope.

Similar content being viewed by others
References
(a) Quin, L. D.; Tyrell, J. A. Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals; John Wiley & Sons: Hoboken, 2010. (b) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257. (c) Joule, J. A.; Mills, K. Heterocyclic Chemistry; Wiley-Blackwell: Chichester, 2010, 5th ed.
Hougaard, C.; Hammami, S.; Eriksen, B. L.; Sørensen, U. S.; Jensen, M. L.; Strøbæk, D.; Christophersen, P. Mol. Pharmacol. 2012, 81, 210.
Gomez, L.; Massari, M. E.; Vickers, T.; Freestone, G.; Vernier, W.; Ly, K.; Xu, R.; McCarrick, M.; Marrone, T.; Metz, M.; Yan, Y. G.; Yoder, Z. W.; Lemus, R.; Broadbent, N. J.; Barido, R.; Warren, N.; Schmelzer, K.; Neul, D.; Lee, D.; Andersen, C. B.; Sebring, K.; Aertgeerts, K.; Zhou, X.; Tabatabaei, A.; Peters, M.; Breitenbucher, J. G. J. Med. Chem. 2017, 60, 2037.
Wang, S.; Zhao, L.-J.; Zheng, Y.-C.; Shen, D.-D.; Miao, E.-F.; Qiao, X.-P.; Zhao, L.-J.; Liu, Y.; Huang, R.; Yu, B.; Liu, H.-M. Eur. J. Med. Chem. 2017, 125, 940.
da Silva, E. R.; Boechat, N.; Pinheiro, L. C. S.; Bastos, M. M.; Costa, C. C. P.; Bartholomeu, J. C.; da Costa, T. H. Chem. Biol. Drug Des. 2015, 86, 969.
Faizi, M.; Dabirian, S.; Tajali, H.; Ahmadi, F.; Zavareh, E. R.; Shahhosseini, S.; Tabatabai, S. A. Bioorg. Med. Chem. 2015, 23, 480.
Arenas-González, A.; Mendez-Delgado, L. A.; Merino-Montiel, P.; Padrón, J. M.; Montiel-Smith, S.; Vega-Báez, J. L.; Meza-Reyes, S. Steroids 2016, 116, 13.
(a) Kokkonda, S.; Deng, X.; White, K. L.; Coteron, J. M.; Marco, M.; de las Heras, L.; White, J.; El Mazouni, F.; Tomchick, D. R.; Manjalanagara, K.; Rudra, K. R.; Chen, G.; Morizzi, J.; Ryan, E.; Kaminsky, W.; Leroy, D.; Santos Martínez-Martínez, M.; Jimenez-Diaz, M. B.; Ferrer Bazaga, S.; Angulo-Barturen, I.; Waterson, D.; Burrows, J. N.; Matthews, D.; Charman, S. A.; Phillips, M. A.; Rathod, P. K. J. Med. Chem. 2016, 59, 5416. (b) Phillips, M. A.; White, K. L.; Kokkonda, S.; Deng, X.; White, J.; El Mazouni, F.; Marsh, K.; Tomchick, D. R.; Manjalanagara, K.; Rudra, K. R.; Wirjanata, G.; Noviyanti, R.; Price, R. N.; Marfurt, J.; Shackleford, D. M.; Chiu, F. C. K.; Campbell, M.; Jimenez-Diaz, M. B.; Ferrer Bazaga, S.; Angulo-Barturen, I.; Santos Martinez, M.; Lafuente-Monasterio, M.; Kaminsky, W.; Silue, K.; Zeeman, A.-M.; Kocken, C.; Leroy, D.; Blasco, B.; Rossignol, E.; Rueckle, T.; Matthews, D.; Burrows, J. N.; Waterson, D.; Palmer, M. J.; Rathod, P. K.; Charman, S. A. ACS Infect. Dis. 2016, 2, 945.
(a) Flynn, J.; Jones, J.; Johnson, A. J.; Andritsos, L.; Maddocks, K.; Jaglowski, S.; Hessler, J.; Grever, M. R.; Im, E.; Zhou, H.; Zhu, Y.; Zhang, D.; Small, K.; Bannerji, R.; Byrd, J. C. Leukemia 2015, 29, 1524 (b) Criscitiello, C.; Viale, G.; Esposito, A.; Curigliano, G. Expert Opin. Invest. Drugs 2014, 23, 13..
(a) Yu, P. B.; Hong, C. C.; Sachidanandan, C.; Babitt, J. L.; Deng, D. Y.; Hoyng, S. A.; Lin, H. Y.; Bloch, K. D.; Peterson, R. T. Nat. Chem. Biol. 2008, 4, 33. (b) Yamamoto, Y.; Sawa, R.; Wake, I.; Morimoto, A.; Okimura, Y. Nutr. Res. 2017, 47, 13.
(a) Yano, W.; Inoue, N.; Ito, S.; Itou, T.; Yasumura, M.; Yoshinaka, Y.; Hagita, S.; Goto, M.; Nakagawa, T.; Inoue, K.; Tanabe, S.; Kaku, K. J. Diabetes Investig. 2017, 8, 155. (b) Ikedo, T.; Minami, M.; Kataoka, H.; Hayashi, K.; Nagata, M.; Fujikawa, R.; Higuchi, S.; Yasui, M.; Aoki, T.; Fukuda, M.; Yokode, M.; Miyamoto, S. J. Am. Heart Assoc. 2017, 6, e004777. (c) Kato, N.; Oka, M.; Murase, T.; Yoshida, M.; Sakairi, M.; Yamashita, S.; Yasuda, Y.; Yoshikawa, A.; Hayashi, Y.; Makino, M.; Takeda, M.; Mirensha, Y.; akigami, T. Bioorg. Med. Chem. 2011, 19, 7221.
(a) El-Gendy, M. M. A.; Shaaban, M.; Shaaban, K. A.; l-Bondkly, A. M.; Laatsch, H. J. Antibiot. 2008, 61, 149. (b) Wang, H.; Hesek, D.; Lee, M.; Lastochkin, E.; Oliver, A. G.; Chang, M.; Mobashery, S. J. Nat. Prod. 2016, 79, 1219.
Ohnishi, H.; Yamaguchi, K.; Shimada, S.; Suzuki, Y.; Kumagai, A. Life Sci. 1981, 28, 1641.
(a) Rodríguez-Torres, M.; Yoshida, E. M.; Marcellin, P.; Srinivasan, S.; Purohit, V. S.; Wang, C.; Hammond, J. L. Ann. Hepatol. 2014, 13, 364. (b) Successful Strategies for the Discovery of Antiviral Drugs; Desai, M. C.; Meanwell, N. A., Eds.; The Royal Society of Chemistry: Cambridge, 2013.
Youssef, Y. A.; Kamel, M. M.; Taher, M. S.; Ali, N. F.; Abd El Megiede, S. A. J. Saudi Chem. Soc. 2014, 18, 220.
(a) Dandia, A.; Khan, S.; Sharma, R.; Parihar, S.; Parewa, V. ChemistrySelect 2017, 2, 9684. (b) Butler, R. N.; Coyne, A. G. Org. Biomol. Chem. 2016, 14, 9945.
(a) Cherukupalli, S.; Karpoormath, R.; Chandrasekaran, B.; Hampannavar, G. A.; Thapliyal, N.; Palakollu, V. N. Eur. J. Med. Chem. 2017, 126, 298. (b) Fizer, M.; Slivka, M. Chem. Heterocycl. Compd. 2016, 52, 155. [Khim. Geterotsikl. Soedin. 2016, 52, 155.]
(a) Sakhno, Y. I.; Kozyryev, A. V; Desenko, S. M.; Shishkina, S. V; Musatov, V. I.; Sysoiev, D. O.; Chebanov, V. A. Tetrahedron 2018, 74, 564. (b) Kumar, P. M.; Kumar, K. S.; Mohakhud, P. K.; Mukkanti, K.; Kapavarapu, R.; Parsa, K. V. L.; Pal, M. Chem. Commun. 2012, 48, 431. (c) Soares de Melo, C.; Feng, T.-S.; van der Westhuyzen, R.; Gessner, R. K.; Street, L. J.; Morgans, G. L.; Warner, D. F.; Moosa, A.; Naran, K.; Lawrence, N.; Boshoff, H. I. M.; Barry III, C. E.; Harris, C. J.; Gordon, R.; Chibale, K. Bioorg. Med. Chem. 2015, 23, 7240. (d) Mokhtar, M.; Saleh, T. S.; Basahel, S. N. J. Mol. Catal. A: Chem. 2012, 353–354, 122. (e) Bartels, B.; Bolas, C. G.; Cueni, P.; Fantasia, S.; Gaeng, N.; Trita, A. S. J. Org. Chem. 2015, 80, 1249. (f) Kaping, S.; Kalita, U.; Sunn, M.; Singha, L. I.; Vishwakarma, J. N. Monatsh. Chem. 2016, 147, 1257. (g) Shekarrao, K.; Kaishap, P. P.; Saddanapu, V.; Addlagatta, A.; Gogoi, S.; Boruah, R. C. RSC Adv. 2014, 4, 24001. (h) Castillo, J.-C.; Rosero, H.-A.; Portilla, J. RSC Adv. 2017, 7, 28483. (i) Farahi, M.; Karami, B.; Banaki, Z. Chin. Chem. Lett. 2015, 26, 1065. (j) Scapin, E.; Salbego, P. R. S.; Bender, C. R.; Meyer, A. R.; Pagliari, A. B.; Orlando, T.; Zimmer, G. C.; Frizzo, C. P.; Bonacorso, H. G.; Zanatta, N.; Martins, M. A. P. Beilstein J. Org. Chem. 2017, 13, 2396. (k) Bharath, Y.; Rao, M. V. B.; Pal, M. Lett. Drug Des. Discovery 2017, 14, 1206. (l) Aggarwal, R.; Kumar, S. Beilstein J. Org. Chem. 2018, 14, 203. (m) Bhatt, J. D.; Chudasama, C. J.; Patel, K. D. Bioorg. Med. Chem. 2015, 23, 7711.
Lancaster, M. Green Chemistry; The Royal Society of Chemistry: Cambridge, 2016, 3rd ed., p. 165.
(a) Anwar, H. F.; Fleita, D. H.; Kolshorn, H.; Meier, H.; Elnagdi, M. H. ARKIVOC 2006, (xv), 133. (b) Al-Matar, H. M.; Khalil, K. D.; Adam, A. Y.; Elnagdi, M. H. Molecules 2010, 15, 6619.
Rahmati, A. C. R. Chim. 2012, 15, 647.
(a) Hassan, A. S.; Mady, M. F.; Awad, H. M.; Hafez, T. S. Chin. Chem. Lett. 2017, 28, 388. (b) Hassan, A. S.; Hafez, T. S.; Osman, S. A. Sci. Pharm. 2015, 83, 27.
(a) Severina, G. I.; Perehoda, L. A.; Georgiyantc, V. A. Pharm. Rev. 2009, 3, 6 [In Ukrainian]. (b) Attaby, F. A.; Eldin, S. M. Arch. Pharm. Res. 1997, 20, 330.
Khurana, J. M.; Nand, B.; Saluja, P. Tetrahedron 2010, 66, 5637.
ACKNOWLEDGEMENT
The authors gratefully acknowledge Mr. Amamudin Ansari for spectroscopic analysis of all synthesized compounds. The authors are thankful to Dr. Jandeep Singh (Assistant Professor, Lovely Professional University, Punjab) for providing help with linguistic corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file, containing IR (compounds 3c, 6b,e, and 7c), 1H and 13C NMR (compounds 3c, 7c,f) spectroscopic and mass spectral data (compounds 3c, 7d), and results of optimization of reaction conditions, is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(3), 246–253
Electronic supplementary material
ESM 1
(PDF 890 kb)
Rights and permissions
About this article
Cite this article
Gol, R.M., Khatri, T.T. & Barot, V.M. Facile Regioselective On-Water Synthesis of 4,7-Dihydropyrazolo[1,5-a]Pyrimidines and 4,7-Dihydro[1,2,4]Triazolo[1,5-a]Pyrimidines. Chem Heterocycl Comp 55, 246–253 (2019). https://doi.org/10.1007/s10593-019-02449-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02449-x