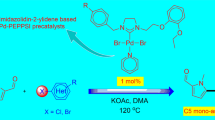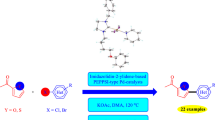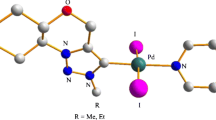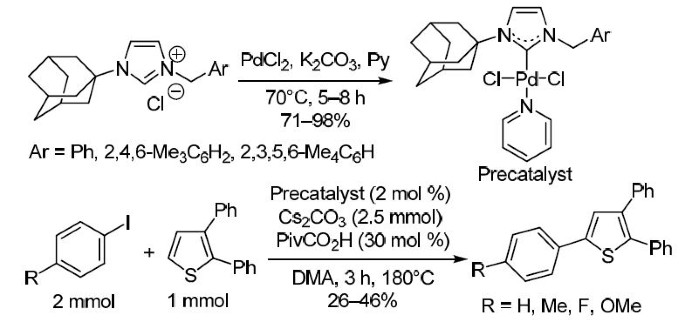
Starting from 1-adamantanyl-3-imidazole, a number of new 1-adamantanyl-3-benzylimidazolium salts and corresponding unsymmetrically substituted Pd(II) PEPPSI-type complexes were prepared, including dichloride, dibromide, and diiodide. Single crystal X-ray crystallography confirmed solid state structures in six cases. The complexes reported in this work displayed moderate activities as precatalysts for CH activation/arylation reaction of substituted thiophenes.














Similar content being viewed by others
References
(a) Herrmann, W. A.; Köcher, C. Angew. Chem., Int. Ed. Engl. 1997, 36, 2162. (b) Herrmann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1290. (c) Hahn, F. E.; Jahnke, M. C. Angew. Chem., Int. Ed. 2008, 47, 3122. (d) Díez-González, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612. (e) Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485. (f) N-Heterocyclic Carbenes. Effective Tools for Organometallic Synthesis; Nolan, S. P., Ed.; WileyVCH, 2014, p. 544. f Nelson, D. J. Eur. J. Inorg. Chem. 2015, 2012.
(a) O'Brien, C. J.; Kantchev, E. A. B.; Valente, C.; Hadei, N.; Chass, G. A.; Lough, A.; Hopkinson, A. C.; Organ, M. G. Chem.–Eur. J. 2006, 12, 4743. (b) Kantchev, E. A. B.; O'Brien, C. J.; Organ, M. G. Angew. Chem., Int. Ed. 2007, 46, 2768. (c) Valente, C.; Çalimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 3314. (d) Valente, C.; Pompeo, M.; Sayah, M.; Organ, M. G. Org. Process Res. Dev. 2014, 18, 180. (e) Diner, C.; Organ, M. G. Organometallics 2019, 38, 66.
(a) Türkmen, H.; Can, R.; Çetinkaya, B. Dalton Trans. 2009, 7039. (b) Li, Y.-J.; Zhang, J.-L.; Li, X.-J.; Geng, Y.; Xu, X.-H.; Jin, Z. J. Organomet. Chem. 2013, 737, 12.
Liu, F.; Zhu, Y.-R.; Song, L.-G.; Lu, J.-M. Org. Biomol. Chem. 2016, 14, 2563.
(a) Batey, R. A.; Shen, M.; Lough, A. J. Org. Lett. 2002, 4, 1411. (b) Linninger, C. S.; Herdtweck, E.; Hoffmann, S. D.; Herrmann, W. A.; Kühn, F. E. J. Mol. Struct. 2008, 890, 192. (c) Tang, Y.-Q.; Lu, J.-M.; Shao, L.-X. J. Organomet. Chem. 2011, 696, 3741. (d) Zhu, L.; Gao, T.-T.; Shao, L.-X. Tetrahedron 2011, 67, 5150. (e) Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. J. Org. Chem. 2012, 77, 6608. (f) Xiao, Z.-K.; Shao, L.-X. Synthesis 2012, 711. (g) Gu, Z.-S.; Shao, L.-X.; Lu, J.-M. J. Organomet. Chem. 2012, 700, 132. (h) Zhu, L.; Ye, Y.-M.; Shao, L.-X. Tetrahedron 2012, 68, 2414. (i) Lin, X.-F.; Li, Y.; Li, S.-Y.; Xiao, Z.-K.; Lu, J.-M. Tetrahedron 2012, 68, 5806. (j) Yin, H.-Y.; Liu, M-.Y.; Shao, L.-X. Org. Lett. 2013, 15, 6042. (k) Yin, S.-C.; Zhou, Q.; He, Q.-W.; Li, S.-W.; Qian, P.-C.; Shao, L.-X. Tetrahedron 2017, 73, 427.
(a) Lv, H.; Zhu, L.; Tang, Y.-Q.; Lu, J.-M. Appl. Organomet. Chem. 2014, 28, 27. (b) Zheng, S.; Wang, Y.; Zhang, C.; Liu, J.; Xia, C. Appl. Organomet. Chem. 2014, 28, 48. (c) Sikorski, W.; Zawartka, W.; Trzeciak, A. M. J. Organomet. Chem. 2018, 867, 323.
(a) Wang, Z.-Y.; Ma, Q.-N.; Li, R.-H.; Shao, L.-X. Org. Biomol. Chem. 2013, 11, 7899.(b) Farmer, J. L.; Pompeo, M; Lough, A. J.; Organ, M. G. Chem.–Eur. J. 2014, 20, 15790.
Türkmen, H., Gök, L.; Kani I.; Çetinkaya, B. Turkish J. Chem. 2013, 37, 633.
(a) Chen, M. T.; Vicic, D. A.; Turner, M. L.; Navarro, O. Organometallics 2011, 30, 5052. (b) Guest, D.; Chen, M.-T.; Tizzard, G. J.; Coles, S. J.; Turner, M. L.; Navarro, O. Eur. J. Inorg. Chem. 2014, 2200. (c) Gallop, C. W. D.; Chen, M.-T.; Navarro, O. Org. Lett. 2014, 16, 3724. (d) Gallop, C. W. D.; Zinser, C.; Guest, D.; Navarro, O. Synlett 2014, 2225.
(a) Randell, K.; Stanford, M. J.; Clarkson, G. J.; Rourke, J. P. J. Organomet. Chem. 2006, 691, 3411. (b) Zheng, S.-Z.; Peng, X.-G.; Liu, J.-M.; Sun, W.; Xia, C.-G. Chin. J. Chem. 2007, 25, 1065. (c) Wang, F.; Zhu, L.; Zhou, Y.; Bao, X.; Shaefer, H. F. III. Chem.–Eur. J. 2015, 21, 4153.
Türkmen, H.; Kani, I. Appl. Organomet. Chem. 2013, 27, 489.
(a) Huang, P.; Wang, Y.-X.; Yu, H.-F.; Lu, J.-M. Organometallics 2014, 33, 1587. (b) Borré, E.; Dahm, G.; Aliprandi, A.; Mauro, M.; Dagorne, S.; Bellemin-Laponnaz, S. Organometallics 2014, 33, 4374. (c) Baier, H.; Metzner, P.; Körzdörfer, T.; Kelling, A.; Holdt, H.-J. Eur. J. Inorg. Chem. 2014, 2952. (d) Wang, Y.; Yang, X.; Zhang, C.; Yu, J.; Liu, J.; Xia, C. Adv. Synth. Catal. 2014, 356, 2539. (e) Dahm, G.; Borré, E.; Guichard, G.; Bellemin-Laponnaz, S. Eur. J. Inorg. Chem. 2015, 1665. (f) Yang, J. Appl. Organomet. Chem. 2017, 31, e3734. (g) Sun, K.-X.; He, Q.-W.; Xu, B.-B.; Wu, X.-T.; Lu, J.-M. Asian J. Org. Chem. 2018, 7, 781.
(a) Gao, T.-T.; Jin, A.-P.; Shao, L.-X. Beilstein J. Org. Chem. 2012, 8, 1916. (b) Lin, Y.-C.; Hsueh, H.-H.; Kanne, S.; Chang, L.-K.; Liu, F.-C.; Lin, I. J. B. Organometallics 2013, 32, 3859. (c) Martínez-Olid, F.; Andrés, R.; Flores, J. C.; Gómez-Sal, P. Eur. J. Inorg. Chem. 2015, 4076.
(a) Organ, M. G.; Çalimsiz, S.; Sayah, M,; Hoi, K. H.; Lough, A. J. Angew. Chem., Int. Ed. 2009, 48, 2383. (b) Teci, M.; Brenner, E.; Matt, D.; Toupet, L. Eur. J. Inorg. Chem. 2013, 2841. (c) Azua, A.; Mata, J. A.; Heymes, P.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E. Adv. Synth. Catal. 2013, 355, 1107. (d) Rajabi, F.; Trampert, J.; Sun, Y.; Busch, M.; Bräse, S.; Thiel, W. R. J. Organomet. Chem. 2013, 744, 101. (e) Benhamou, L.; Besnard, C.; Kündig, E. P. Organometallics 2014, 33, 260. (f) Teci, M.; Brenner, E.; Matt, D.; Gourlaouen, C.; Toupet, L. Dalton Trans. 2015, 44, 9260. (g) Yaşar, S.; Şahin, Ҫ.; Arslan, M.; Özdemir, İ. J. Organomet. Chem. 2015, 776, 107. (h) Groomridge, B. J.; Goldup, S. M.; Larossa, I. Chem. Commun. 2015, 51, 3832. (i) Baier, H.; Kelling, A.; Holdt, H.-J. Eur. J. Inorg. Chem. 2015, 1950. (j) Akkoç, S.; Gök, Y.; İlhan, İ. Ö.; Kayser, V. Beilstein J. Org. Chem. 2016, 12, 81. (k) Lei, P.; Meng, G.; Ling, Y.; An, J.; Szostak, M. J. Org. Chem. 2017, 82, 6638. (l) Lu, D.-D.; He, X.-X.; Liu, F.-S. J. Org. Chem. 2017, 82, 10898. (m) Chernenko, A. Yu.; Astakhov, A. V.; Pasyukov, D. V.; Dorovatovskii, P. V.; Zubavichus, Ya. V.; Khrustalev, V. N.; Chernyshev, V. M. Russ. Chem. Bull. 2018, 67, 79. [Izv. Akad. Nauk, Ser. Khim. 2018, 79.]
(a) Yang, L.; Li, Y.; Chen, Q.; Du, Y.; Cao, C.; Shi, Y.; Pang, G. Tetrahedron 2013, 69, 5178. o Dahm, G.; Bailly, C.; Karmazin, L.; Bellemin-Laponnaz, S. J. Organomet. Chem. 2015, 794, 115.
(a) Çalimsiz, S.; Sayah, M.; Mallik, D.; Organ, M. G. Angew. Chem., Int. Ed. 2010, 49, 2014. (b) Pompeo, M.; Froese, R. D. J.; Hadei, N.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 11354. (c) Liu, Z.; Dong, N.; Xu, M.; Sun, Z.; Tu, T. J. Org. Chem. 2013, 78, 7436. (d) Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Pompeo, M.; Froese, R. D. J.; Organ, M. G. Angew. Chem., Int. Ed. 2015, 54, 9502. (e) Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Organ, M. G. Chem.–Eur. J. 2016, 22, 14531.
(a) Organ, M. G.; Abdel-Hadi, M.; Avola, S.; Dubovyk, I.; Hadei, N.; Kantchev, E. A. B.; O'Brien, C. J.; Sayah, M. Chem.–Eur. J. 2008, 14, 2443. (b) Chartoire, A.; Frogneux, X.; Boreux, A.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2012, 31, 6947. (c) Chen, W.-X.; Shao, L.-X. J. Org. Chem. 2012, 77, 9236. (d) Hoi, K. H.; Coggan, J. A.; Organ, M. G. Chem.–Eur. J. 2013, 19, 843. (e) Fang, W.; Jiang, J.; Xu, Y.; Zhou, J.; Tu, T. Tetrahedron 2013, 69, 673. (f) Krinsky, J. L.; Martínez, A.; Godard, C.; Castillón, S.; Claver, C. Adv. Synth. Catal. 2014, 356, 460. (g) Zhang, Y.; César, V.; Storch, G.; Lugan, N.; Lavigne, G. Angew. Chem., Int. Ed. 2014, 53, 6482. (h) Zhang, Y.; César, V.; Lavigne, G. Eur. J. Org. Chem. 2015, 2042. (i) Lombardi, C.; Day, J.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Farmer, J. L.; Organ, M. G. Organometallics 2017, 36, 251. (j) Khadra, A.; Mayer, S.; Organ, M. G. Chem.–Eur. J. 2017, 23, 3206. (k) Shi, S.; Szostak, M. Chem. Commun. 2017, 53, 10584. (l) Huang, F.-D.; Xu, C.; Lu, D.-D.; Shen, D.-S.; Li, T.; Liu, F.-S. J. Org. Chem. 2018, 83, 9144.
Sayah, M.; Lough, A. J.; Organ, M. G. Chem.–Eur. J. 2013, 19, 2749.
(a) He, P.; Du, Y.; Wang, S.; Cao, C.; Wang, X.; Pang, G.; Shi, Y. Z. Anorg. Allg. Chem. 2013, 639, 1004. (b) Xiap, Z.-K.; Yin, H.-Y.; Shao, L.-X. Org. Lett. 2013, 15, 1254. (c) Shen, X.-B.; Zhang, Y.; Chen, W.-X.; Xiao, Z.-K.; Hu, T.-T.; Shao, L.-X. Org. Lett. 2014, 16, 1984. (d) Gu, Z.-S.; Chen, W.-X.; Shao, L.-X. J. Org. Chem. 2014, 79, 5806. (e) Akkoç, S.; Gök, Y. Inorg. Chim. Acta. 2015, 429, 34. (f) Akkoç, S.; Gök, Y.; İlhan, İ. Ö.; Kayser, V. Beilstein J. Org. Chem. 2016, 12, 81. (g) Marelli, E.; Corpet, M.; Minenkov, Yu.; Neyyappadath, R. M.; Bismuto, A.; Buccolini, G.; Curcio, M.; Cavallo, L.; Nolan, S. P. ACS Catal. 2016, 6, 2930. (h) He, X.-X.; Li, Y.; Ma, B.-B.; Ke, Z.; Liu, F.-S. Organometallics 2016, 35, 2655. (i) Hu, L.-Q.; Deng, R.-L.; Li, Y.-F.; Zeng, C.-J.; Shen, D.-S.; Liu, F.-S. Organometallics 2018, 37, 214. (j) Liu, F.; Hu, Y.-Y.; Li, D.; Zhou, Q.; Lu, J.-M. Tetrahedron 2018, 74, 5683. (k) Panyam, P. K. R.; Ugale, B.; Gandhi, T. J. Org. Chem. 2018, 83, 7622. (l) Kaloğlu, N.; Kaloğlu, M.; Tahir, M. N.; Arici, C.; Bruneau, C.; Doucet, H.; Dixneuf, P. H.; Çetinkaya, B.; Özdemir, İ. J. Organomet. Chem. 2018, 867, 404. (m) Chen, W.; Yang, J. J. Organomet. Chem. 2018, 872, 24.
Zheng, S.; Peng, X.; Liu, J.; Sun, W.; Xia, C. Helv. Chim. Acta 2007, 90, 1471.
(a) Tornatzky, J.; Kannenberg, A.; Blechert, S. Dalton Trans. 2012, 41, 8215. (b) Queval, P.; Jahier, C.; Rouen, M.; Artur, I.; Legeay, J.-C.; Falivene, L.; Toupet, L.; Crévisy, C.; Cavallo, L.; Baslé, O.; Mauduit, M. Angew. Chem., Int. Ed. 2013, 52, 14103. (c) Mangold, S. L.; O'Leary, D. J.; Grubbs, R. H. J. Am. Chem. Soc. 2014, 136, 12469. (d) Paradiso, V.; Bertolasi, V.; Costabile, C.; Grisi, F. Dalton Trans. 2016, 45, 561. (e) Tarrieu, R.; Dumas, A.; Thongpaen, J.; Vives, T.; Roisnel, T.; Dorcet, V.; Crévisy, C.; Baslé, O.; Mauduit, M. J. Org. Chem. 2017, 82, 1880. (f) Box, H. K.; Howell, T. O.; Kennon, W. E.; Burk, G. A.; Valle, H. U.; Hollis, T. K. Tetrahedron. 2017, 73, 2191. (g) Paradiso, V.; Bertolasi, V.; Costabile, C.; Caruso, T.; Dąbrowski, M.; Grela, K.; Grisi, F. Organometallics 2017, 36, 3692. (h) Pape, F.; Teichert, J. F. Eur. J. Org. Chem. 2017, 4206.
(a) Ray, L.; Shaikh, M. M.; Ghosh, P. Dalton Trans. 2007, 4546. (b) Ray, L.; Barman, S.; Shaikh, M. M.; Ghosh, P. Chem.–Eur. J. 2008, 14, 6646. (c) Fang, W.; Jiang, J.; Xu, Y.; Zhou, J.; Tu, T. Tetrahedron 2013, 69, 673. (d) Yang, L.; Li, Y.; Chen, Q.; Du, Y.; Cao, C.; Shi, Y.; Pang, G. Tetrahedron 2013, 69, 5178. (e) Lin, Y.-C.; Hsueh, H.-H.; Kanne, S.; Chang, L.-K.; Liu, F.-C.; Lin, I. J. B. Organometallics 2013, 32, 3859. (f) Zheng, S.; Wang, Y.; Zhang, C.; Liu, J.; Xia, C. Appl. Organomet. Chem. 2014, 28, 48. (g) Benhamou, L.; Besnard, C.; Kündig, E. P. Organometallics 2014, 33, 260. (h) Rouen, M.; Borré, E.; Falivene, L.; Toupet, L.; Berthod, M.; Cavallo, L.; Olivier-Borbigou, H.; Maudit, M. Dalton Trans. 2014, 43, 7044. (i) Osińska, M.; Gniewek, A.; Trzeciak, A. M. J. Mol. Catal. A: Chem. 2016, 418–419, 9. (j) Topchiy, M. A.; Zotova, M. A.; Masoud, S. M.; Mailyan, A. K.; Ananyev, I. V.; Nefedov, S. E.; Asachenko, A. F.; Osipov, S. N. Chem.–Eur. J. 2017, 23, 6663. (k) Halter, O.; Plenio, H. Eur. J. Inorg. Chem. 2018, 2935.
Agnew-Francis, K. A.; Williams, C. M. Adv. Synth. Catal. 2016, 358, 675.
Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E. A. B.; O'Brien C. J.; Lough, A.; Organ, M. G. Chem.–Eur. J. 2010, 16, 10844.
(a) Zeiler, A.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Chem.–Eur. J. 2015, 21, 11065. (b) Bolbat, E.; Wendt, O. F. Eur. J. Org. Chem. 2016, 3395. (c) Shao, L.; Lu, J.; Liu, F. CN Patent 106892945A.
(a) Arduengo, A. J., III; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361. (b) Gstöttmayr, C. W. K.; Böhm, V. P. W.; Herdweck, E.; Grosche, M.; Herrmann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1363.
(a) Queval, P.; Jahier, C.; Rouen, M.; Artur, I.; Legeay, J.-C.; Falivene, L.; Toupet, L.; Crévisy, C.; Cavallo, L.; Baslé, O.; Mauduit, M. Angew. Chem., Int. Ed. 2013, 52, 14103. (b) Tarrieu, R.; Dumas, A.; Thongpaen, J.; Vives, T.; Roisnel, T.; Dorcet, V.; Crévisy, C.; Baslé, O.; Mauduit, M. J. Org. Chem. 2017, 82, 1880. (c) Dumas, A.; Tarrieu, R.; Vives, T.; Roisnel, T.; Dorcet, V.; Baslé, O.; Mauduit, M. ACS Catal. 2018, 8, 3257.
Alcalde, E.; Mesquida, N.; Alemany, M.; Alvares-Rúa, C.; García-Granda, S.; Pacheco, P.; Pérez-Garcia, L. Eur. J. Org. Chem. 2002, 1221.
Glushkov, V. A.; Arapov, K. A.; Kotelev, M. S.; Rudowsky, K. S.; Suponitsky, K. Yu.; Gorbunov, A. A.; Maiorova, O. A.; Slepukhin, P. A. Heteroat. Chem. 2012, 23, 5.
Glushkov, V. A.; Zhiguleva, M. A.; Maiorova, O. A.; Gorbunov, A. A. Russ. J. Org. Chem. 2012, 48, 699. [Zh. Org. Khim. 2012, 48, 701.]
(a) Ray, L.; Shaikh, M. M.; Ghosh, P. Dalton Trans. 2007, 4546. (b) Liao, C.-Y.; Chan, K.-T.; Zeng, J.-Y.; Hu, C.-H.; Tu, C.-Y.; Lee, H. M. Organometallics 2007, 26, 1692. (c) Yang, L.; Zhao, J.; Li, Y.; Ge, K.; Zhuang, Y.; Cao, C.; Shi, Y. Inorg. Chem. Commun. 2012, 22, 33.
(a) Dunsford, J. J.; Cavell, K. J. Organometallics 2014, 33, 2902. (b) Dahm, G.; Bailly, C.; Karmazin, L.; BelleminLaponnaz, S. J. Organomet. Chem. 2015, 794, 115. (c) Lan, X.-B.; Li, Y.; Li, Y.-F.; Shen, D.-S.; Ke, Z.; Liu, F.-S. J. Org. Chem. 2017, 82, 2914.
Dash, C.; Shaikh, M. M.; Ghosh, P. Eur. J. Inorg. Chem. 2009, 1608.
(a) Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174. (b) Gorelsky, S. I.; Lapointe, D.; Fagnou, K. J. Org. Chem. 2012, 77, 658. (c) Steinmetz, M.; Ueda, K.; Grimme, S.; Yamaguchi, J.; Kirchberg, S.; Itami, K.; Studer, A. Chem.– Asian J. 2012, 7, 1256. (d) Fu, H.; Chen, H.; Doucet, H. Appl. Organomet. Chem. 2013, 27, 595. (e) Jiang, H.; Bellomo, A.; Zhang, M.; Carroll, P. J.; Manor, B. C.; Jia, T.; Walsh, P. J. Org. Lett. 2018, 20, 2522.
(a) Steinmetz, M.; Ueda, K.; Grimme, S.; Yamaguchi, J.; Kirchberg, S.; Itami, K.; Studer, A. Chem.–Asian J. 2012, 7, 1256. (b) Shibahara, F.; Asai, Y.; Murai, T. Asian J. Org. Chem. 2018, 7, 1323.
(a) Okazawa, T.; Satoh, T.; Miura, M.; Nomura. M. J. Am. Chem. Soc. 2002, 124, 5286. (b) Nakano, M.; Tsurugi, H.; Satoh, T.; Miura, M. Org. Lett. 2008, 10, 1851.
Kaloğlu, M.; Özdemir, I.; Dorcet, V.; Bruneau, C.; Doucet, H. Eur. J. Inorg. Chem. 2017, 1382.
(a) Gorelsky, S. I.; Lapointe, D.; Fagnou, K. J. Am. Chem. Soc. 2008, 130, 10848. (b) Liégault, B.; Lapointe, D.; Caron, L.; Vlassova, A.; Fagnou, K. J. Org. Chem. 2009, 74, 1826. (c) Gorelsky, S. I.; Lapointe, D.; Fagnou, K. J. Org. Chem. 2012, 77, 658.
(a) de Vries, J. G. Dalton Trans. 2006, 421. (b) Zhang, W.; Qi, H.; Li, L.; Wang, X.; Chen, J.; Peng, K.; Wang, Z. Green Chem. 2009, 11, 1194. (c) Tsvelikhovsky, D.; Popov, I.; Gutkin, V.; Rozin, A.; Shvartsman, A.; Blum, J. Eur. J. Org. Chem. 2009, 98. (d) Ohtaka, A.; Tamaki, Y.; Igawa, Y.; Egami, K.; Shimomura, O.; Nomura, R. Tetrahedron 2010, 66, 5642. (e) Balanta, A.; Godard, C.; Claver, C. Chem. Soc. Rev. 2011, 40, 4973. (f) Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Chem. Soc. Rev. 2011, 40, 5181. (g) Wang, W.; Yang, Q.; Zhou, R.; Fu, H.-Y.; Li, R.-X.; Chen, H.; Li, X.-J. J. Organomet. Chem. 2012, 697, 1. (h) Gildner, P. G.; Colacot, T. J. Organometallics 2015, 34, 5497.
Astakhov, A. A.; Khazipov, O. V.; Chernenko, A. Yu.; Pasyukov, D. V.; Kashin, A. S.; Gordeev, E. G.; Khrustalev, V. N.; Chernyshev, V. M.; Ananikov, V. P. Organometallics 2017, 36, 1981.
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
Würtz, S.; Glorius, F. Acc. Chem. Res. 2008, 41, 1523.
(a) Froese, R. D. J.; Lombardi, C.; Pompeo, M.; Rucker, R. P.; Organ, M. G. Acc. Chem. Res. 2017, 50, 2244. (b) Zhang, Y.; Lavigne, G.; Lugan, N.; César, V. Chem.–Eur. J. 2017, 23, 13792.
(a) Horton, A. W. J. Org. Chem. 1949, 14, 761. (b) Voronkov, M. G.; Brown, A. S.; Karpenko, A. S.; Gol'shtein, B. L. Zh. Obshch. Khim. 1949, 19, 1356. (c) Oae, S; Ishihara, H.; Yoshihara, M. Chem. Heterocycl. Compd. 1995, 31, 917. [Khim. Geterotsikl. Soedin. 1995, 1053.]
Voronkov, M. G.; Udre, V.; Taube, A. Chem. Heterocycl. Compd. 1971, 7, 703. [Khim. Geterotsikl. Soedin. 1971, 755.] 46. (a) Relyea, D. I.; Hubbard, W. L.; Grahame, R. E., Jr. DE Patent 2724494A1, 1977. (b) Gotthardt, Н.; Weisshuhn, C. M. Chem. Ber. 1978, 111, 2028.
(a) Relyea, D. I.; Hubbard, W. L.; Grahame, R. E., Jr. DE Patent 2724494A1, 1977. (b) Gotthardt, Н.; Weisshuhn, C. M. Chem. Ber. 1978, 111, 2028.
(a) Kang, K-T.; Lee, S. J.; Jyung, K. K. J. Korean Chem. Soc. 1998, 42, 584. (b) Ueda, K.; Yanagisawa, S.; Yamaguchi, J.; Itami, K. Angew. Chem., Int. Ed. 2010, 49, 8946.
Shklyaeva, E. V.; Shklyaev, Yu. V. SU Patent 1395636, 1988,.
Voronkov, M. G.; Gol'shtein, B. L. Zh. Obshch. Khim. 1950, 20, 1218.
Nagano, T.; Kimoto, H.; Nakatsuji, H.; Motoyoshiya, J.; Aoyama, H.; Tanabe, Y.; Nishii, Y. Chem. Lett. 2007, 36, 62.
Raenko, G. F.; Korotkikh, N. I.; Pekhtereva, T. M.; Shvaika, O. P. Russ. J. Org. Chem. 2001, 37, 1153. [Zh. Org. Khim. 2001, 37, 1212.]
(a) Pfeffer, M.; Braunstein, P.; Dehand, J. Spectrochim. Acta, Part A 1974, 30A, 331. (b) Mostafa Hossain, A. G. M.; Nagaoka, T.; Ogura, K. Electrochim. Acta 1996, 41, 2773. (c) Biagini, M. C.; Ferrari, M.; Lanfranchi, M.; Marchiò, L.; Pellinghelli, M. A. J. Chem. Soc., Dalton Trans. 1999, 1575. (d) Pazderski, L.; Tousek, J.; Sitkowski, J.; Malinakova, K.; Kozerski, L.; Szlyk, E. Magn. Reson. Chem 2009, 47, 228.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Farrugia, L. J. J. Appl. Crystallogr. 2012, 45, 849.
Sheldrick, G. M. Acta Crystallogr., Sect A: Found. Crystallogr. 2008, A64, 112.
Sheldrick, G. M. Acta Crystallogr., Sect C: Struct. Chem. 2015, C71, 3.
Spek, A. L. Acta Crystallogr., Sect C: Struct. Chem. 2015, С71, 9.
This work was supported by Russian Foundation for Basic Research (grant 17-03-00456-a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(3), 217–228
Electronic supplementary material
ESM 1
(PDF 20672 kb)
Rights and permissions
About this article
Cite this article
Glushkov, V.A., Denisov, M.S., Gorbunov, A.A. et al. Adamantanyl-substituted PEPPSI-type palladium(II) N-heterocyclic carbene complexes: synthesis and catalytic application for CH activation of substituted thiophenes. Chem Heterocycl Comp 55, 217–228 (2019). https://doi.org/10.1007/s10593-019-02445-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02445-1