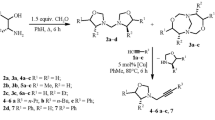
An efficient one-pot synthetic procedure for [4-(tert-butyl)-1H-pyrrol-3-yl](phenyl)methanone was elaborated using acetophenone and trimethylacetaldehyde in the presence of TosMIC and mild base LiOH·H2O. This method is very economical for the synthesis of pyrrole derivatives and was successfully utilized with good yields.


Similar content being viewed by others
References
(a) Sbardella, G.; Mai, A.; Artico, M.; Loddo, R.; Setzu, M. G.; La Colla, P. Bioorg. Med. Chem. Lett. 2004, 14, 1537. (b) Raimondi, M. V.; Cascioferro, S.; Schillaci, D.; Petruso, S. Eur. J. Med. Chem. 2006, 41, 1439. (c) Biava, M.; Porretta, G. C.; Poce, G.; Deidda, D.; Pompei, R.; Tafi, A.; Manetti, F. Bioorg. Med. Chem. 2005, 13, 1221.
Di Santo, R.; Tafi, A.; Costi, R.; Botta, M.; Artico, M.; Corelli, F.; Forte, M.; Caporuscio, F.; Angiolella, L.; Palamara, A. T. J. Med. Chem. 2005, 48, 5140.
Bialer, M.; Yagen, B.; Mechoulam, R.; Becker, Y. J. Med. Chem. 1980, 23, 1144.
(a) Dannhardt, G.; Kiefer, W.; Krämer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. Eur. J. Med. Chem. 2000, 35, 499. (b) Laufer, S. A.; Augustin, J.; Dannhardt, G.; Kiefer, W. J. Med. Chem. 1994, 37, 1894. (c) Khanna, I. K.; Weier, R. M.; Yu, Y.; Collins, P. W.; Miyashiro, J. M.; Koboldt, C. M.; Veenhuizen, A. W.; Currie, J. L.; Seibert, K.; Isakson, P. C. J. Med. Chem. 1997, 40, 1619.
(a) Wakabayashi, K.-i.; Imai, K.; Miyachi, H.; Hashimoto, Y.; Tanatani, A. Bioorg. Med. Chem. 2008, 16, 6799. (b) Lee, H.; Lee, J.; Shin, Y.; Jung, W.; Kim, J.-H.; Park, K.; Ro, S.; Chung, H.-H.; Koh, J. S. Bioorg. Med. Chem. Lett. 2001, 11, 2963.
Artico, M; Di Santo, R.; Costi, R.; Massa, S.; Retico, A.; Artico, M.; Apuzzo, G.; Simonetti, G.; Strippoli, V. J. Med. Chem. 1995, 38, 4223.
Tafi, A.; Costi, R.; Botta, M.; Di Santo, R.; Corelli, F.; Massa, S.; Ciacci, A.; Manetti, F.; Artico, M. J. Med. Chem. 2002, 45, 2720.
(a) Harrington, P. E.; Tius, M. A. Org. Lett. 1999, 1, 649. (b) Sun, H.; Zhang, H.; Ang, E. L.; Zhao, H. Bioorg. Med. Chem. 2018, 26, 1275. (c) Honey; Thareja, S.; Kumar, M.; Sinha, V. R. Eur. J. Med. Chem. 2012, 53, 76.
Rapoport, H.; Harbuck, J. W. J. Org. Chem. 1971, 36, 853.
Amarnath, V.; Anthony, D. C.; Amarnath, K.; Valentine, W. M.; Wetterau, L. A.; Graham, D. G. J. Org. Chem. 1991, 56, 6924.
(a) Knorr, L. Ber. Dtsch. Chem. Ges. 1884, 17, 1635. (b) Knorr, L. Justus Liebigs Ann. Chem. 1886, 236, 290. (c) Cheng, L.; Lightner, D. A. Synthesis 1999, 46. (d) Lavecchia, A.; Costi, R.; Artico, M.; Miele, G.; Novellino, E.; Bergamini, A.; Crespan, E.; Maga, G.; Di Santo, R. ChemMedChem 2006, 1, 1379.
(a) Hantzsch, A. Ber. 1890, 23, 1474. (b) Feist, F. Ber. 1902, 35, 1537. (c) Trautwein, A. W.; Süßmuth, R. D.; Jung, G. Bioorg. Med. Chem. Lett. 1998, 8, 2381. (d) Roomi, M. W.; MacDonald, S. F. Can. J. Chem. 1970, 48, 1689.
(a) Paal, C. Ber. 1885, 18, 367. (b) Knorr, L. Ber. 1885, 18, 299. (c) Minetto, G.; Raveglia, L. F.; Sega, A.; Taddei, M. Eur. J. Org. Chem. 2005, 5277. (d) Rao, H. S. P.; Jothilingam, S.; Scheeren, H. W. Tetrahedron 2004, 60, 1625.
(a) Garabatos-Perera, J. R.; Rotstein, B. H.; Thompson, A. J. Org. Chem. 2007, 72, 7382. (b) The Chemistry of Pyrroles; Jones, R. A.; Bean, G. P., Eds.; Academic Press: London, 1977, p. 525.
Thompson, A.; Butler, R. J.; Grundy, M. N.; Laltoo, A. B. E.; Robertson, K. N.; Cameron, T. S. J. Org. Chem. 2005, 70, 3753.
Van Leusen, A. M.; Hoogenboom, B. E.; Houwing, H. A. J. Org. Chem. 1976, 41, 711.
Possel, O.; Van Leusen, A. M. Tetrahedron Lett. 1977, 18, 4229.
Oldenziel, O. H.; Van Leusen, A. M. Tetrahedron Lett. 1974, 15, 167.
(a) Kumar, V.; Nair, V. A. Tetrahedron Lett. 2010, 51, 966. (b) Khatik, G. L.; Pal, A.; Mobin, S. M.; Nair, V. A. Tetrahedron Lett. 2010, 51, 3654. (c) Chouhan, M.; Sharma, R.; Nair, V. A. Appl. Organomet. Chem. 2011, 25, 470.
(a) Chouhan, M.; Kumar, K.; Sharma, R.; Grover, V.; Nair, V. A. Tetrahedron Lett. 2013, 54, 4540. (b) Goyal, S.; Patel, J. K.; Gangar, M.; Kumar, K.; Nair, V. A. RSC Adv. 2015, 5, 3187. (c) Kumar, V.; Kumar, K.; Pal, A.; Khatik, G. L.; Nair, V. A. Tetrahedron 2013, 69, 1747. (d) Kumar, K.; Mudshinge, S. R.; Goyal, S.; Gangar, M.; Nair, V. A. Tetrahedron Lett. 2015, 56, 1266. (e) Chouhan, M.; Senwar, K. R.; Kumar, K.; Sharma, R.; Nair, V. A. Synthesis 2014, 195. (f) Sharma, R.; Kumar, K.; Chouhan, M.; Grover, V.; Nair, V. A. RSC Adv. 2013, 3, 14521.
(a) Goyal, S.; Patel, B.; Sharma, R.; Chouhan, M.; Kumar, K.; Gangar, M.; Nair, V. A. Tetrahedron Lett. 2015, 56, 5409. (b) Kumar, K.; More, S. S.; Goyal, S.; Gangar, M.; Khatik, G. L.; Rawal, R. K.; Nair, V. A. Tetrahedron Lett. 2016, 57, 2315. (c) Kumar, K.; Siddique, J.; Gangar, M.; Goyal, S.; Rawal, R. K.; Nair, V. A. ChemistrySelect 2016, 1, 2409. (d) Kumar, K.; Konar, D.; Goyal, S.; Gangar, M.; Chouhan, M.; Rawal, R. K.; Nair, V. A. ChemistrySelect 2016, 1, 3228. (e) Kumar, K.; Konar, D.; Goyal, S.; Gangar, M.; Chouhan, M.; Rawal, R. K.; Nair, V. A. J. Org. Chem. 2016, 81, 9757. (f) Kumar, K.; More, S. S.; Khatik, G. L.; Rawal, R. K.; Nair, V. A. J. Heterocycl. Chem. 2017, 54, 2696. (g) Kaur, R.; Manjal, S. K.; Rawal, R. K.; Kumar, K. Bioorg. Med. Chem. 2017, 25, 4533. (h) Manjal, S. K.; Kaur, R.; Bhatia, R.; Kumar, K.; Singh, V.; Shankar, R.; Kaur, R.; Rawal, R. K. Bioorg. Chem. 2017, 75, 406.
(a) Mittal, M.; Kumar, K.; Anghore, D.; Rawal, R. K. Curr. Drug Discovery Technol. 2017, 14, 106. (b) Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M. K.; Rawal, R. K. Eur. J. Med. Chem. 2017, 132, 108. (c) Kumar, B.; Singh, V.; Shankar, R.; Kumar, K.; Rawal, R. K. Curr. Top. Med. Chem. 2017, 17, 148. (d) Talwan, P.; Chaudhary, S.; Kumar, K.; Rawal, R. K. Curr. Bioact. Compd. 2017, 13, 109. (e) Kaur, R.; Rani, V.; Abbot, V.; Kapoor, Y.; Konar, D.; Kumar, K. J. Pharm. Chem. Chem. Sci. 2017, 1, 17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(7), 700–702
Rights and permissions
About this article
Cite this article
Kaur, R., Kumar, K. One-pot synthesis of [4-(tert-butyl)-1H-pyrrol-3-yl](phenyl)methanone from tosylmethyl isocyanide and carbonyl compound. Chem Heterocycl Comp 54, 700–702 (2018). https://doi.org/10.1007/s10593-018-2335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2335-6