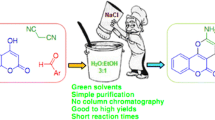
New chromene derivatives, 9H-furo[2,3-f]chromene-7,8-dicarboxylates, have been synthesized in high yields by a three-component reaction of euparin (isolated from Petasites hybridus), an aldehyde, and dialkyl acetylenedicarboxylate in the presence of PPh3 and catalytic amount of ZnO nanorods under solvent-free conditions at room temperature. ZnO nanorods showed a strong effect on improving the yield of the desired products. Chromene derivatives can be easily separated from the reaction mixture in a simple work-up, and the catalyst may be recovered and reused without considerable loss of activity.


Similar content being viewed by others
References
Tao, J. A.; Kazlauskas, R. J. Biocatalysis for Green Chemistry and Chemical Process Development; John Wiley & Sons, 2011.
(a) Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, 2005. (b) Orru, R. V. A.; Ruijter, E. Synthesis of Heterocycles via Multicomponent Reactions II; Springer: Berlin, 2010. (c) Dӧmling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
Ellis, G. P. The Chemistry of Heterocyclic Compounds: Chromenes, Chromanones, and Chromones; Wiley-Interscience: Ontario, 2009.
Cassidy, F.; Evans, J. M.; Hadley, M. S.; Haladij, A. H.; Leach, P. E.; Stemp, G. J. Med. Chem. 1992, 35, 1623.
Robertson, D. W.; Steinberg, M. I. J. Med. Chem. 1990, 33, 1529.
Lee, B. H.; Seo, H. W.; Yoo, S.-E. Pharmacology 2004, 70, 74.
Mori, J.; Iwashima, M.; Takeuchi, M.; Saito, H. Chem. Pharm. Bull. 2006, 54, 391.
Engler, T. A.; LaTessa, K. O.; Iyengar, R.; Chai, W.; Agrios, K. Bioorg. Med. Chem. 1996, 4, 1755.
Hafez, H. N.; Hegab, M. I.; Ahmed-Farag, I. S.; El-Gazzar, A. B. A. Bioorg. Med. Chem. Lett. 2008, 18, 4538.
Ronad, P.; Dharbamalla, S.; Hunshal, R.; Maddi, V. Arch. Pharm. 2008, 341, 696.
Starks, C. M.; Williams, R. B.; Norman, V. L.; Rice, S. M.; O'Neil-Johnson, M.; Lawrence, J. A.; Eldridge, G. R. Phytochemistry 2014, 98, 216.
Gӧrlitzer, K.; Dehne, A.; Engler, E. Arch. Pharm. 1983, 316, 264.
(a) Xu, Z.-Q.; Pupek, K.; Suling, W. J.; Enache, L.; Flavin, M. T. Bioorg. Med. Chem. 2006, 14, 4610. (b) Jeso, V.; Nicolaou, K. C. Tetrahedron Lett. 2009, 50, 1161.
Brunavs, M.; Dell, C. P.; Gallagher, P. T.; Owton, W. M.; Smith, C. W. EU Patent 557075; Chem. Abstr. 1994, 120, 1067681.
(a) Alvey, L.; Prado, S.; Huteau, V.; Saint-Joanis, B.; Michel, S.; Koch, M.; Cole, S. T.; Tillequin, F.; Janin,Y. L. Bioorg. Med. Chem. 2008, 16, 8264. (b) Symeonidis, T.; Chamilos, M.; Hadjipavlou-Litina, D. J.; Kallitsakis, M.; Litinas, K. E. Bioorg. Med. Chem. Lett. 2009, 19, 1139.
Eiden, F.; Denk, F. Arch. Pharm. 1991, 324, 353.
Hafez, E. A. A.; Elnagdi, M. H.; Elagamey, A. G. A.; El-Taweel, F. M. A. A. Heterocycles 1987, 26, 903.
Schweizer, E. E.; Meeder-Nycz, D. In The Chemistry of Heterocyclic Compounds; Ellis, G. P., Ed.; John Wiley: New York, 2008, vol. 31, p. 11.
(a) Zonouzi, A.; Googheri, M. S.; Miralinaghi, P. S. Org. Prep. Proced. Int. 2008, 40, 471. (b) Zonouzi, A.; Izakian, Z.; Biniaz, M. Org. Prep. Proced. Int. 2009, 41, 543. (c) Zonouzi, A.; Biniaz, M.; Mirzazadeh, R.; Talebi, M.; Ng, S. W. Heterocycles 2010, 81, 1271. (d) Zonouzi, A.; Hosseinzadeh, F.; Karimi, N.; Mirzazadeh, R.; Ng, S. W. ACS Comb. Sci. 2013, 15, 240.
(a) Sabbaghan, M.; Hossaini, Z. Comb. Chem. High Throughput Screening 2012, 15, 745. (b) Hosseini-Sarvari, M.; Sharghi, H.; Etemad, S. Helv. Chim. Acta 2008, 91, 715. (c) Shaterian, H. R.; Mohammadnia, M. J. Mol. Liq. 2013, 177, 353.
(a) Rostamizadeh, S.; Nojavan, M.; Aryan, R.; Isapoor, E.; Azad, M. J. Mol. Catal. A: Chem. 2013, 374-375, 102. (b) Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. J. Nanopart. Res. 1999, 1, 439.
(a) Comparelli, R.; Fanizza, E.; Curri, M. L.; Cozzoli, P. D.; Mascolo, G.; Agostiano, A. Appl. Catal., B 2005, 60, 1. (b) Moghaddam, F. M.; Saeidian, H. Mater. Sci. Eng., B 2007, 139, 265. (c) Mirjafary, Z.; Saeidian, H.; Sadeghi, A.; Moghaddam, F. M. Catal. Commun. 2008, 9, 299. (d) Gupta, M.; Paul, S.; Gupta, R.; Loupy, A. Tetrahedron Lett. 2005, 46, 4957. (e) Lietti, L.; Tronconi, E.; Forzatti, P.; Busca, G. J. Mol. Catal. 1989, 55, 43. (f) Eskandari, K.; Karami, B.; Khodabakhshi, S. Chem. Heterocycl. Compd. 2015, 50, 1658. [Khim. Geterotsikl. Soedin. 2014, 1807.]
Khaleghi, F.; Bin Din, L.; Rostami Charati, F.; Yaacob, W. A.; Khalilzadeh, M. A.; Skelton, B.; Makha, M. Phytochem. Lett. 2011, 4, 254.
(a) Lipton, R. B.; Göbel, H.; Einhäupl, K. M.; Wilks, K.; Mauskop, A. Neurology 2004, 63, 2240. (b) Husain, F.; Pardo, G.; Rabadi, M. Curr. Treat. Options Neurol. 2018, 20, 10.
(a) Hossaini, Z.; Ghambarian, M.; Afshari Sharif Abad, S.; Mohtat, B. Lett. Org. Chem. 2015, 12, 176. (b) Hossaini, Z.; Rostami-Charati, F.; Ghasemian, M.; Afshari Sharif Abad, S. Synlett 2015, 1222. (c) Hossaini, Z.; Sheikholeslami-Farahani, F.; Rostami-Charati, F. Comb. Chem. High Throughput Screening 2014, 17, 804.
(a) Sabbaghan, M.; Anaraki Firooz, A.; Jan Ahmadi, V. J. Mol. Liq. 2012, 175, 135. (b) Mehrabi Matin, B.; Mortazavi, Y.; Khodadadi, A. A.; Abbasi, A.; Anaraki Firooz, A. Sens. Actuators, B 2010, 151, 140.
Hosseini-Sarvari, M.; Tavakolian, M. Appl. Catal., A 2012, 441-442, 65.
Hudson, H. In The Chemistry of Organophosphorus Compounds: Primary, Secondary and Tertiary Phosphines and Heterocyclic Organophosphorus(III) Compounds; Hartley, F. R.; Patai, S., Eds.; Wiley: New York, 1990, p. 386.
Engel, R.; Cohen, J. I. Synthesis of Carbon–Phosphorus Bonds; CRC Press: Boca Raton, 1998.
Cadogan, J. I. G. Organophosphorus Reagents in Organic Synthesis; Academic Press: New York, 1979.
Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863.
Kolodiazhynyi, O. I. Russ. Chem. Rev. 1997, 66, 225. [Usp. Khim. 1977, 66, 246.]
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(6), 593–597
Rights and permissions
About this article
Cite this article
Sheikholeslami-Farahani, F., Karami, H., Salimifard, M. et al. ZnO nanorod-catalyzed three-component reaction of euparin, aldehyde, and dialkyl acetylenedicarboxylate: green synthesis of chromene derivatives. Chem Heterocycl Comp 54, 593–597 (2018). https://doi.org/10.1007/s10593-018-2313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2313-z