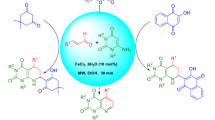
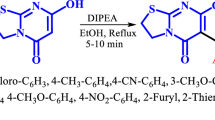
A microwave-assisted Biginelli-like three-component condensation using salicylic aldehyde derivatives, acetone, and 5-substituted 3-amino-1,2,4-triazoles instead of the urea component results in the formation of oxygen-bridged tetrahydrotriazolopyrimidine derivatives (11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5-c][1,3,5]benzoxadiazocines) in good yields and high purity. A plausible reaction mechanism for this transformation is discussed in details using literature and experimental data.

Similar content being viewed by others
References
(a) Biginelli, P. Gazz. Chim. Ital. 1891, 21, 487. (b) Tron, G. C.; Minassi, A.; Appendino, G. Eur. J. Org. Chem. 2011, 5541.
(a) Kappe, C. O. Tetrahedron 1993, 49, 6937. (b) Suresh; Sandhu, J. S. ARKIVOC 2012, (i), 66. (c) Heravi, M. M.; Asadi, S.; Lashkariani, B. M. Mol. Diversity 2013, 17, 389. (d) Barbero, M.; Cadamuro, S.; Dughera, S. Green Chem. 2017, 19, 1529.
(a) Panda, S. S.; Khanna, P.; Khanna, L. Curr. Org. Chem. 2012, 16, 507. (b) Alvim, H. G. O.; Lima, T. B.; de Oliveira, A. L.; de Oliveira, H. C. B.; Silva, F. M.; Gozzo, F. C.; Souza, R. Y.; da Silva, W. A.; Neto, B. A. D. J. Org. Chem. 2014, 79, 3383.
(a) Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043. (b) Slobbe, P.; Ruijter, E.; Orru, R. V. A. MedChemComm 2012, 3, 1189. (c) de Fátima, Â.; Braga, T. C.; Neto, L. d. S.; Terra, B. S.; Oliveira, B. G. F.; da Silva, D. L.; Modolo, L. V. J. Adv. Res. 2015, 6, 363. (d) Sepehri, S.; Sanchez, H. P.; Fassihi, A. J. Pharm. Pharm. Sci. 2015, 18, 1.
(a) Dondoni, A.; Massi, A. Acc. Chem. Res. 2006, 39, 451. (b) Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444. (c) Wan, J.-P.; Liu, Y. Synthesis 2010, 3943.
(a) Svetlik, J.; Hanus, V.; Bella, J. J. Chem. Res., Synop. 1991, 4. (b) Abbas, E. M. H.; Abdallah, S. M.; Abdoh, M. H.; Tawfik, H. A.; El-Hamouly, W. S. Turk. J. Chem. 2008, 32, 297. (c) Světlík, J.; Veizerová, L.; Kettmann, V. Tetrahedron Lett. 2008, 49, 3520. (d) El-Hamouly, W. S.; Tawfik, H. A.; Abbas, E. M. H. Green Chem. Lett. Rev. 2009, 2, 213. (e) Cheng, Q.; Wang, Q.; Xu, X.; Ruan, M.; Yao, H.; Yang, X. J. Heterocycl. Chem. 2010, 47, 624.
(a) Chebanov, V. A.; Gura, K. A.; Desenko, S. M. Top. Heterocycl. Chem. 2010, 23, 41. (b) Chebanov, V. A.; Desenko, S. M. Diversity-Oriented Synth. 2012, 1, 43.
(a) Svetlik, J.; Veizerová, L.; Mayer, T. U.; Catarinella, M. Bioorg. Med. Chem. Lett. 2010, 20, 4073. (b) Světlík, J.; Prónayová, N.; Švorc, L.; Frecer, V. Tetrahedron 2014, 70, 8354. (c) Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019. (d) Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481. (e) Svĕtlík, J.; Prónayová, N.; Frecer, V.; Cież, D. Tetrahedron 2016, 72, 7620.
Sedash, Y. V.; Gorobets, N. Yu.; Chebanov, V. A.; Konovalova, I. S.; Shishkin, O. V.; Desenko, S. M. RSC Adv. 2012, 2, 6719.
(a) Gorobets, N. Yu.; Sedash, Y. V.; Ostras, K. S.; Zaremba, O. V.; Shishkina, S. V.; Baumer, V. N.; Shishkin, O. V.; Kovalenko, S. M.; Desenko, S. M.; Van der Eycken, E. V. Tetrahedron Lett. 2010, 51, 2095. (b) Kondratiuk, M.; Gorobets, N. Yu.; Sedash, Y. V.; Gümüş, M. K.; Desenko, S. M. Molbank 2016, 2016, M898.
Komykhov, S. A.; Bondarenko, A. A.; Musatov, V. I.; Diachkov, M. V.; Gorobets, N. Yu.; Desenko, S. M. Chem. Heterocycl. Compd. 2017, 53, 378. [Khim. Geterotsikl. Soedin. 2017, 53, 378.]
Světlík, J.; Kettmann, V. Tetrahedron Lett. 2011, 52, 1062.
Frolova, L. V.; Malik, I.; Uglinskii, P. Y.; Rogelj, S.; Kornienko, A.; Magedov, I. V. Tetrahedron Lett. 2011, 52, 6643.
Claridge, T. D. W.; Davies, S. G.; Polywka, M. E. C.; Roberts, P. M.; Russell, A. J.; Savory, E. D.; Smith, A. D. Org. Lett. 2008, 10, 5433.
Chen, Q.; Jiang, L.-L.; Chen, C.-N.; Yang, G.-F. J. Heterocycl. Chem. 2009, 46, 139.
(a) Kappe, C. O. J. Org. Chem. 1997, 62, 7201. (b) Alvim, H. G. O.; da Silva Júnior, E. N.; Neto, B. A. D. RSC Adv. 2014, 4, 54282.
(a) Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen'ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389. (b) Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoyev, D. O.; Groth, U.; Kappe, C. O.; Chebanov, V. A. Tetrahedron 2008, 64, 11041.
Gümüş, M. K.; Gorobets, N. Yu.; Sedash, Y. V.; Shishkina, S. V.; Desenko, S. M. Tetrahedron Lett. 2017, 58, 3446.
Cieplak, A. S. In Structure Correlation; Bürgi, H. B.; Dunitz, J. D., Eds.; Wiley-VCH: Weinheim, 2008, p. 205.
Gilmore, K.; Alabugin, I. V. Chem. Rev. 2011, 111, 6513.
Světlík, J.; Tureček, F.; Hanuš, V. J. Chem. Soc., Perkin Trans. 1 1987, 563.
The work was supported by Artvin Coruh University research project (BAP-2012.F19.02.24) and the Council of Higher Education of Turkey, Mevlana Exchange Program (MEV-2016-027).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing 1H and 13C NMR spectral data of the obtained compounds 11a–w is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(11), 1261–1267
Electronic supplementary material
ESM 1
(PDF 3891 kb)
Rights and permissions
About this article
Cite this article
Gümüş, M.K., Gorobets, N.Y., Sedash, Y.V. et al. A modified Biginelli reaction toward oxygen-bridged tetrahydropyrimidines fused with substituted 1,2,4-triazole ring. Chem Heterocycl Comp 53, 1261–1267 (2017). https://doi.org/10.1007/s10593-018-2204-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2204-3