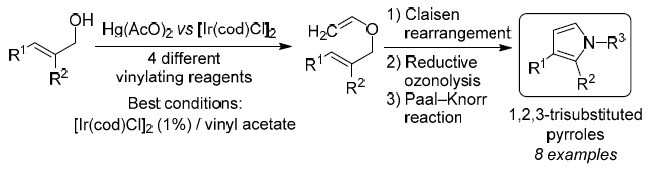
An efficient method for the preparation of 1,3-disubstituted and 1,2,3-trisubstituted pyrroles was developed through a sequence involving O-vinylation of allyl alcohols followed by Claisen rearrangement and further ozonolysis, concurring in a Paal–Knorr reaction with primary amines. Hg(II) and Ir(I) catalysts and different vinylating reagents were tested. The best results were consistently obtained with the [Ir(cod)Cl]2–vinyl acetate system. The featured methodology gives access to functionalized pyrroles in overall good yields, whose chemical architecture may awake interest for assorted applications.

Similar content being viewed by others
References
(a) Young, I. S.; Thornton, P. D.; Thompson, A. Nat. Prod. Rep. 2010, 27, 1801. (b) Seiple, I. B.; Su, S.; Young, I. S.; Lewis, C. A.; Yamaguchi, J.; Baran, P. S. Angew. Chem., Int. Ed. 2010, 49, 1095.
Seidel, D.; Lynch, V.; Sessler, J. L. Angew. Chem., Int. Ed. 2002, 41, 1422.
Walsh, C. T.; Garneau-Tsodikova, S.; Howard-Jones, A. R. Nat. Prod. Rep. 2006, 23, 517.
(a) Anzenbacher, P.; Nishiyabu, R.; Palacios, M. A. Coord. Chem. Rev. 2006, 250, 2929. (b) Domingo, V. M.; Alemán, C.; Brillas, E.; Juliá, L. J. Org. Chem. 2001, 66, 4058.
(a) Seth, B. K.; Ray, A.; Saha, A.; Saha, P.; Basu, S. J. Photochem. Photobiol., B 2014, 132, 72. (b) MacLean, P. D.; Chapman, E. E.; Dobrowolski, S. L.; Thompson, A.; Barclay, L. R. C. J. Org. Chem. 2008, 73, 6623.
(a) Battilocchio, C.; Poce, G.; Alfonso, S.; Porretta, G. C.; Consalvi, S.; Sautebin, L.; Pace, S.; Rossi, A.; Ghelardini, C.; Mannelli, L. D. C.; Schenone, S.; Giordani, A.; Francesco, L. D.; Patrignani, P.; Biava, M. Bioorg. Med. Chem. 2013, 21, 3695. (b) Mohamed, M. S; Kamel, R.; Fathallah, S. S. Arch. Pharm. (Weinheim, Ger.) 2011, 344, 830.
(a) Baran, P. S.; Richter, J. M.; Lin, D. W. Angew. Chem., Int. Ed. 2005, 44, 609. (b) Vyas, S.; Trivedi, P.; Chaturvedi, S. Acta Pharm. (Zagreb, Croatia) 2007, 57, 441.
(a) Hilmy, K. M. H.; Soliman, D. H.; Shahin, E. B. A.; Alhameed, R. A. Life Sci. J. 2012, 9, 736. (b) Edwards, T. G.; Koeller, K. J.; Slomczynska, U.; Fok, K.; Helmus, M.; Bashkin, J. K.; Fisher, C. Antivir. Res. 2011, 91, 177.
(a) Idhayadhulla, A.; Kumar, R. S.; Nasser, A. J. A.; Manilal, A. Bull. Chem. Soc. Ethiop. 2012, 26, 429. (b) El-Sayed, R.; Khairou, K. S. J. Surfactants Deterg. 2015, 18, 661.
Gupton, J. T. Top. Heterocycl. Chem. 2006, 2, 53.
(a) Schneider, J. G.; Eynatten, M.; Parhofer, K. G.; Volkmer, J. E.; Schiekofer, S.; Hamann, A.; Nawroth, P. P.; Dugi, K. A. Atherosclerosis (Amsterdam, Neth.) 2004, 175, 325. (b) Krause, B. R.; Newton, R. S. Atherosclerosis 1995, 117, 237.
(a) Balme, G. Angew. Chem., Int. Ed. 2004, 43, 6238. (b) Jacobi, P. A.; Coutts, L. D.; Guo, J.; Hauck, S. I.; Leung, S. H. J. Org. Chem. 2000, 65, 205. (c) Khan, A. T.; Lal, M.; Bagdi, P. R.; Basha, R. S.; Saravanan, P.; Patra, S. Tetrahedron Lett. 2012, 53, 4145. (d) Reddy, G. R.; Reddy, T. R.; Joseph, S. C.; Reddy, K. S.; Reddy, L. S.; Kumar, P. M.; Krishna, G. R.; Reddy, C. M.; Rambabu, D.; Kapavarapu, R.; Lakshmi, C.; Meda, T.; Priya, K.; Parsa, K. V. L.; Pal, M. Chem. Commun. 2011, 47, 7779.
(a) Reddy, V. P.; Kumar, A. V.; Rao, K. R. Tetrahedron Lett. 2011, 52, 777. (b) Yan, R.-L.; Luo, J.; Wang, C.-X.; Ma, C.-W.; Huang, G.-S.; Liang, Y.-M. J. Org. Chem. 2010, 75, 5395. (c) Tamaddon, F.; Farahi, M.; Karami, B. J. Mol. Catal. A: Chem. 2012, 356, 85.
Elming, N.; Clauson-Kaas, N. Acta Chem. Scand. 1952, 6, 867.
(a) Veisi, H. Tetrahedron Lett. 2010, 51, 2109. (b) Cadierno, V.; Gimeno, J.; Nebra, N. Chem.–Eur. J. 2007, 13, 9973.
(a) Humenny, W. J.; Kyriacou, P.; Sapeta, K.; Karadeolian, A.; Kerr, M. A. Angew. Chem. 2012, 124, 11250. (b) Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2010, 39, 4402.
Michlik, S.; Kempe, R. Nat. Chem. 2013, 5, 140.
Kolos, N. N.; Zubar, V. V.; Omelchenko, I. V.; Musatov, V. I. Chem. Heterocycl. Compd. 2016, 52, 237. [Khim. Geterotsikl. Soedin. 2016, 52, 237.]
Babaei, S. E.; Hossaini, Z.; Besheli, R. R.; Tavakkoli, V. Chem. Heterocycl. Compd. 2016, 52, 294. [Khim. Geterotsikl. Soedin. 2016, 52, 294.]
Haraguchi, K.; Takahashi, H.; Shiina, N.; Horii, C.; Yoshimura, Y.; Nishikawa, A.; Sasakura, E.; Nakamura, K. T.; Tanaka, H. J. Org. Chem. 2002, 67, 5919.
(a) Zhang, F.; Du, P.; Chen, J.; Wang, H.; Luo, Q.; Wan, X. Org. Lett. 2014, 16, 1932. (b) Yoo, E.-J.;Wasa, M.;Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 17378.
(a) Buchi, G.; Vogel, D. E. J. Org. Chem. 1985, 50, 4664. (b) Morita, M.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 2006, 71, 6285. (c) Ooi, T.; Takahashi, M.; Yamada, M.; Tayama, E.; Omoto, K.; Maruoka, K. J. Am. Chem. Soc. 2004, 126, 1150. (d) Wei, X.; Lorenz, J. C.; Kapadia, S.; Saha, A.; Haddad, N.; Busacca, C. A.; Senanayake, C. H. J. Org. Chem. 2007, 72, 4250.
Watanabe, W. H.; Conlon, L. E. J. Am. Chem. Soc. 1957, 79, 2828.
Okimoto, Y.; Sakaguchi, S.; Ishii, Y. J. Am. Chem. Soc. 2002, 124, 1590.
(a) Banik, B. K.; Banik, I.; Renteria, M.; Dasgupta, S. K. Tetrahedron Lett. 2005, 46, 2643. (b) Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213.
(a) Méndez, J. M.; Flores, B.; León, F.; Martínez, M. E.; Vázquez, A.; García, G. A.; Salmón, M. Tetrahedron Lett. 1996, 37, 4099. (b) Kagabu, S.; Tsuji, H.; Kawai, I.; Ozeki, H. Bull. Chem. Soc. Jpn. 1995, 68, 341.
Thanks are given to Facultad de Química, UNAM for financial support and also CONACYT for scholarship to D. A. R. (CV/grant number 387462/255265). As well, we want to thank Dr. Rafael Omar Arcos Ramos and Professor Joseph Muchowski for the corrections to early manuscripts of this work. Deep gratitude is also expressed to the USAII division from Facultad de Química, UNAM and Dr. Rosa Santillan Baca for the acquisition of spectroscopic data and MS experiments, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(5), 526–531
Rights and permissions
About this article
Cite this article
Alavez-Rosas, D., Maldonado-Domínguez, M., González-Antonio, O. et al. Synthesis of 1,3- and 1,2,3-functionalized pyrroles via Ir(I)-catalyzed vinylation of allyl alcohols. Chem Heterocycl Comp 53, 526–531 (2017). https://doi.org/10.1007/s10593-017-2087-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2087-8