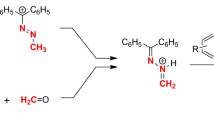
Dihetaryl thioketones possessing thiophen-2-yl and selenophen-2-yl rings react as “superdienophilic” reagents with nonactivated 1,3-dienes such as 2,3-dimethylbuta-1,3-diene, cyclopentadiene, and mixtures of isomeric hexa-2,4-dienes to produce the expected 2H-thiopyrans in moderate to excellent yields. In the latter case, the corresponding cis-2,2-dihetaryl-3,6-dimethyl-3,6-dihydro-2H-thiopyrans are formed as the sole products in a stereoconvergent thia-Diels–Alder reaction. A stepwise mechanism via delocalized diradical intermediates is postulated to rationalize the observed reaction course. Treatment of 4,5-dimethyl-2,2-di(thiophen-2-yl)-3,6-dihydro-2H-thiopyran with excess of m-CPBA at room temperature leads to the oxidation of the C=C bond and the sulfur atom in the six-membered ring.

Similar content being viewed by others
Change history
05 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10593-021-02954-y
References
Blond, G.; Gulea, M.; Mamane, V. Curr. Org. Chem. 2016, 20, 2161.
(a) Block, E. In Science of Synthesis, Molander, G. A., Ed.; G. Thieme Verlag: Stuttgart, 2007, vol. 33, ch. 33, p. 235. (b) Tavakolinia, F.; Baghipour, T.; Hossaini, Z.; Zareyee, D.; Mohammad, A.; Khalilzadeh, M. A.; Rajabi, M. Nucleic Acid Ther. 2012, 22, 265.
(a) Bogdanowicz-Szwed, K.; Budzowski, A. Wiad. Chem. 2003, 57, 435 (PL-ISSN 0043-5104). (b) Mlostoń, G.; Grzelak, P.; Heimgartner, H. J. Sulfur Chem. 2017, 38, 1.
Timoshenko, V. M.; Siry, S. A.; Rozhenko, A. B.; Shermolovich, Y. G. J. Fluorine Chem. 2010, 131, 172.
Dentel, H.; Chataigner, I.; Lohier, J.-F.; Gulea, M. Tetrahedron 2012, 68, 2326.
(a) Kanishchev, O. S.; Sanselme, M.; Bouillon, J.-P. Tetrahedron 2013, 69, 1322. (b) Mikhailichenko, S. S.; Bouillon, J.-P.; Besson, T.; Shermolovich, Y. G. Tetrahedron Lett. 2010, 51, 990.
Larsen, C.; Harpp, D. N. J. Org. Chem. 1980, 45, 3713.
(a) Schönberg, A.; König, B. Chem. Ber. 1968, 101, 725. (b) König, B.; Martens, J.; Praefcke, K.; Schönberg, A.; Schwarz, H.; Zeisberg, R. Chem. Ber. 1974, 107, 2931. (c) Praefcke, K.; Weichsel, C. Liebigs Ann. Chem. 1979, 784. (d) Weinreb, S. M.; Staib, R. R. Tetrahedron 1982, 38, 3087. (e) Metzner, P. Synthesis 1992, 1185. (f) Itoh, T.; Fujikawa, K.; Kubo, M. J. Org. Chem. 1996, 61, 8329. (g) Mlostoń, G.; Grzelak, P.; Hamera-Fałdyga, R.; Jasiński, M.; Pipiak, P.; Urbaniak, K.; Albrecht, Ł.; Hejmanowska, J.; Heimgartner, H. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 204.
(a) Baumann, K.; Fromm, E. Ber. Dtsch. Chem. Ges. 1889, 22, 2593. (b) Gaumont, A. C.; Wazneh, L.; Denis, J. M. Tetrahedron 1991, 47, 4927.
(a) Middleton, W. J. J. Org. Chem. 1965, 30, 1390. (b) Schuler, B.; Sundermeyer, W. Chem. Ber. 1990, 123, 177.
(a) Larsen, S. D.; Fisher, P. V.; Libby, B. E.; Jensen, R. M.; Mizsak, S. A.; Watt, W. J. Org. Chem. 1996, 61, 4725. (b) Huang, N.-Z.; Lakshmikantham, M. V.; Cava, M. P. J. Org. Chem. 1987, 52, 169.
(a) Sasaki, T.; Eguchi, S.; Katada, T. Heterocycles 1984, 21, 682. (b) Katada, T.; Eguchi, S.; Sasaki, T. J. Org. Chem. 1986, 51, 314.
(a) Bonini, B. F.; Mazzanti, G.; Zani, P.; Maccagnani, G. J. Chem. Soc., Perkin Trans. 1 1989, 2083. (b) Bonini, B. F.; Lenzi, A.; Maccagnani, G.; Barbaro, G.; Giorgianni, P.; Macciantelli, D. J. Chem. Soc., Perkin Trans. 1 1987, 2643.
(a) Ito, S.; Okujima, T.; Kikuchi, S.; Shoji, T.; Morita, N.; Asao, T.; Ikoma, T.; Tero-Kubota, S.; Kawakami, J.; Tajiri, A. J. Org. Chem. 2008, 73, 2256. (b) Breu, J.; Höcht, P.; Rohr, U.; Schatz, J.; Sauer, J. Eur. J. Org. Chem. 1998, 2861.
(a) Schatz, J.; Sauer, J. Tetrahedron Lett. 1994, 35, 4767. (b) Rohr, U.; Schatz, J.; Sauer, J. Eur. J. Org. Chem. 1998, 2875.
Kiselev, V. D.; Kashaeva, E. A.; Potapova, L. N.; Iskhakova, G. G.; Konovalov, A. I. Russ. Chem. Bull., Int. Ed. 2005, 54, 2034. [Izv. Akad. Nauk, Ser. Khim. 2005, 1973.]
Jiang, H.; Cruz Cruz, D.; Li, Y.; Lauridsen, V. H.; Jørgensen, K. A. J. Am. Chem. Soc. 2013, 135, 5200.
Hejmanowska, J.; Jasiński, M.; Mlostoń, G.; Albrecht, Ł. Eur. J. Org. Chem. 2017, 950.
Mlostoń, G.; Urbaniak, K.; Gębicki, K.; Grzelak, P.; Heimgartner, H. Heteroat. Chem. 2014, 25, 548.
Hamera-Fałdyga, R.; Grzelak, P.; Pipiak, P.; Mlostoń, G. Phosphorus, Sulfur Silicon Relat. Elem. 2017, 192, 197.
Varma, R. S.; Kumar, D. Org. Lett. 1999, 1, 697.
Wilker, S.; Erker, G. J. Am. Chem. Soc. 1995, 117, 10922.
(a) Mlostoń, G.; Urbaniak, K.; Linden, A.; Heimgartner, H. Helv. Chim. Acta 2015, 98, 453. (b) Mlostoń, G.; Pipiak, P.; Linden, A.; Heimgartner, H. Helv. Chim. Acta 2015, 98, 462. (c) Mlostoń, G.; Pipiak, P.; Heimgartner, H. Beilstein J. Org. Chem. 2016, 12, 716. (d) Mlostoń, G.; Hamera-Fałdyga, R.; Linden, A.; Heimgartner, H. Beilstein J. Org. Chem. 2016, 12, 1421.
Johnson, C. K. ORTEPII, Report ORNL-5138: Oak Ridge National Laboratory: Oak Ridge, Tennessee, 1976.
Praefcke, K.; Weichsel, C. Liebigs Ann. Chem. 1980, 1604.
Li, H.; Silver, J. E.; Watson, W. H.; Kashyap, R. P.; Le Noble, W. J. J. Org. Chem. 1991, 56, 5932.
(a) Petrov, V. A.; Lustig, S.; Marshall, W. J. Fluorine Chem. 2007, 128, 1227. (b) Schwab, M.; Sundermeyer, W. Chem. Ber. 1986, 119, 2458.
Block, E.; Wall, A. Tetrahedron Lett. 1985, 26, 1425.
Alder, K.; Vogt, W. Liebigs Ann. Chem. 1951, 571, 137.
Magnusson, G. J. Org. Chem. 1985, 50, 1998.
Biellmann, J. F.; Ducep, J. B.; Vicens, J. J. Tetrahedron 1976, 32, 1801.
CrysAlisPro, Version 1.171.38.43c; Rigaku Oxford Diffraction: Abingdon, 2015.
Sheldrick, G. M. Acta Crystallogr. Sect. A 2015, 71, 3.
Parsons, S.; Flack, H. D.; Wagner, T. Acta Crystallogr. Sect. B 2013, 69, 249.
Maslen, E. N.; Fox, A. G.; O'Keefe, M. A. In International Tables for Crystallography, Wilson, A. J. C., Ed.; Kluwer Academic Publishers: Dordrecht, 1992, vol. C, Table 6.1.1.1, p. 477.
Stewart, R. F.; Davidson, E. R.; Simpson, W. T. J. Chem. Phys. 1965, 42, 3175.
Ibers, J. A.; Hamilton, W. C. Acta Crystallogr. 1964, 17, 781.
Creagh, D. C.; McAuley, W. J. In International Tables for Crystallography; Wilson, A. J. C., Ed.; Kluwer Academic Publishers: Dordrecht, 1992, vol. C, Table 4.2.6.8, p. 219.
Creagh, D. C.; Hubbell, J. H. In International Tables for Crystallography; Wilson, A. J. C., Ed.; Kluwer Academic Publishers: Dordrecht, 1992, vol. C, Table 4.2.4.3, p. 200.
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, C71, 3.
The authors thank the National Science Center (Cracow, PL) for financial support within the project Maestro (Grant Number: Dec-2012/06/A/ST5/00219). This work is a part of the planned PhD thesis of Paulina Grzelak, University of Łódź.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(5), 518–525
Rights and permissions
About this article
Cite this article
Mlostoń, G., Grzelak, P., Linden, A. et al. Thia-Diels–Alder reactions of hetaryl thioketones with nonactivated 1,3-dienes leading to 3,6-dihydro-2H-pyrans: evidence for a diradical mechanism. Chem Heterocycl Comp 53, 518–525 (2017). https://doi.org/10.1007/s10593-017-2086-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2086-9