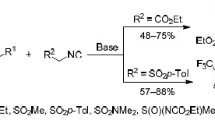
Multicomponent reactions of ethyl 1-trifluoromethyl-2-(trimethylsilyloxy)cyclopropanecarboxylate, isonitriles, and either 2-aminopyridine or amino acids provided imidazo[1,2-a]pyridines or γ-lactams, respectively. This access to trifluoromethyl-substituted heterocycles demonstrates again that siloxycyclopropanes can serve as hidden functionalized aldehydes and that this type of donor–acceptor-substituted cyclopropanes is particularly versatile.

Similar content being viewed by others
Notes
Signals marked with an asterisk were assigned to the minor diastereomers.
References
(a) Reissig, H.-U. Top. Curr. Chem. 1988, 144, 73. (b) Reissig, H.-U.; Zimmer, R. Chem. Rev. 2003, 103, 1151.
Recent reviews: (a) Schneider, T. F.; Kaschel, J.; Werz, D. B. Angew. Chem., Int. Ed. 2014, 53, 5504; Angew. Chem. 2014, 126, 5608. (b) For a collection of other reviews on various aspects of D–A cyclopropanes, see Isr. J. Chem. 2016, 56, 365.
(a) Ugi, I.; Lohberger, S.; Karl, R. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon Press: Oxford, 1991, vol. 2, chap. 4.6. (b) Ugi, I. J. Prakt. Chem. 1997, 339, 499. (c) Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168; Angew. Chem. 2000, 118, 3300. (d) Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem.–Eur. J. 2000, 6, 3321. (e) Zhu, J. Eur. J. Org. Chem. 2003, 1133. (f) Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, 2005. (g) Dömling, A. Chem. Rev. 2006, 106, 17. (h) Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083. (i) Zarganes-Tzitzkas, T.; Chandgude, A. L; Dömling, A. Chem. Rec. 2015, 15, 981.
(a) Zimmer, R.; Ziemer, A.; Gruner, M.; Brüdgam, I.; Hartl, H.; Reissig, H.-U. Synthesis 2001, 1649. (b) Veljkovic, I.; Zimmer, R.; Reissig, H.-U.; Brüdgam, I.; Hartl, H. Synthesis 2006, 2677.
(a) Gladow, D.; Reissig, H.-U. Synthesis 2013, 2179. (b) Gladow, D.; Reissig, H.-U. J. Org. Chem. 2014, 79, 4492. (c) Gladow, D.; Doniz-Kettenmann, S.; Reissig, H.-U. Helv. Chim. Acta 2014, 97, 808.
(a) Gladow, D.; Reissig, H.-U. Helv. Chim. Acta 2012, 95, 1818. (b) Laub, H. A.; Gladow, D.; Reissig, H.-U.; Mayr, H. Org. Lett. 2012, 14, 3990.
(a) Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661. (b) Blackborn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Tetrahedron Lett. 1998, 39, 3635. (c) Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. 1998, 37, 2234; Angew. Chem. 1998, 110, 2349. (d) Mandair, G. S.; Light, M.; Russell, A.; Hursthouse, M.; Bradley, M. Tetrahedron Lett. 2002, 43, 4267. (e) Lyon, M. A.; Kercher, T. S. Org. Lett. 2004, 6, 4989. Recent reviews: (f) Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183. (g) Shaaban, S.; Abdel-Wahab, B. F. Mol. Diversity 2016, 20, 233.
(a) Paquette, L. A.; Crich, D.; Fuchs, P. L.; Molander, G. A. Encyclopedia of Reagents for Organic Synthesis; Wiley: Chichester, 2009, 2nd ed., p. 4086. (b) Carpino, L. A. J. Am. Chem. Soc. 1993, 115, 4397.
Salwiczek, M.; Nyakatura, E. K.; Gerling, U. I. M.; Ye, S.; Koksch B. Chem. Soc. Rev. 2012, 41, 2135.
(a) Demharter, A.; Hörl, W.; Herdtweck, E.; Ugi, I. Angew. Chem., Int. Ed. Engl. 1996, 35, 173; Angew. Chem. 1996, 108, 185. (b) Dawidowski, M.; Herold, F.; Wilczek, M.; Turlo, J.; Chodkowski, A.; Gomolka, A.; Kleps, J. Tetrahedron 2012, 68, 8222. (c) Dawidowski, M.; Sobczak, S.; Wilczek, M.; Kulesza, A.; Turlo, J. Mol. Diversity 2014, 18, 61.
(a) Lubineau, A. J. Org. Chem. 1986, 51, 2142. (b) Pirrung, M. C.; Sarma, K. D. J. Am. Chem. Soc. 2003, 126, 444.
Farrugia, L. J. J. Appl. Crystallogr. 1997, 30, 565.
Gewald reactions involving siloxycyclopropanes: (a) Özbek, H.; Veljkovic, I.; Reissig, H.-U. Synlett 2008, 3145. (b) Özbek, H.; Lentz, D.; Reissig, H.-U. Eur. J. Org. Chem. 2010, 6319.
Jung, K. E.; Kang, Y. K.; Kim, D.; Park, S. Arch. Pharm. Res. 1997, 20, 346.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 59.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(4), 416–421
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 486 kb)
Rights and permissions
About this article
Cite this article
Gladow, D., Senf, D., Wiecko, J. et al. New trifluoromethyl-substituted heterocycles by multicomponent reactions of siloxycyclopropanes. Chem Heterocycl Comp 53, 416–421 (2017). https://doi.org/10.1007/s10593-017-2068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2068-y