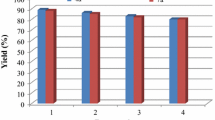
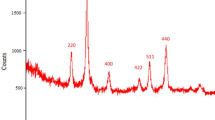
Magnetic Fe3O4 nanoparticles as a heterogeneous catalyst were found to be efficient for the synthesis of a series of pyrrole and indole derivatives using three-component reactions of primary amines, 1,3-dicarbonyl compounds, and activated acetylenic compounds under solvent-free conditions at room temperature. The present protocol offers the advantages of clean reaction, short reaction time, high yield, easy purification, and affordability of the catalyst.


Similar content being viewed by others
References
Li, C.-J.; Chan, T.-H. Comprehensive Organic Reactions in Aqueous Media; John Wiley & Sons: Hoboken, 2007, 2nd ed.
Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725.
Breslow, R. Acc. Chem. Res. 1991, 24, 159.
Samai, S.; Nandi, G. C.; Kumar, R.; Singh, M. S. Tetrahedron Lett. 2009, 50, 7096.
Dömling, A.; Ugi, I. A. Angew. Chem., Int. Ed. 2000, 39, 3168.
Simon, C.; Constantieux, T.; Rodriguez, J. Eur. J. Org. Chem. 2004, 2004, 4957.
Hu, P.; Long, M. Appl. Catal. B: Environ. 2016, 181, 103.
Rateb, N. M.; Elnagdy, S. M.; Zohdi, H. F. Int. J. Adv. Res. 2014, 2, 355.
Radhakrishan, R.; Do, D. M.; Jaenicke, S.; Sasson, Y.; Chuah, G. K. ACS Catal. 2011, 1, 1631.
Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Fuel 2008, 87, 2798.
Ebrahimzadeh, H.; Moazzen, E.; Amini, M. M.; Sadeghi, O. Anal. Methods 2012, 4, 3232.
Esmaeilpour, M.; Sardarian, A. R.; Javidi, J. Appl. Catal., A 2012, 445–446, 359.
Reisser, M.; Maas, G. J. Org. Chem. 2004, 69, 4913.
Sundberg, R. J. In Comprehensive Heterocyclic Chemistry II; Katritzky, A., Rees, C. W., Scriven, E. F. V., Eds.; Pergamon: Oxford, 1996, Vol. 2, p. 119.
Ghabraie, E.; Balalaie, S.; Bararjanian, M.; Bijanzadeh, H. R.; Rominger, F. Tetrahedron 2011, 67, 5415.
Yurovskaya, M. A.; Alekseyev, R. S. Chem. Heterocycl. Compd. 2014, 49, 1400. [Khim. Geterotsikl. Soedin. 2013, 1507.]
Kalinin, A. A.; Mamedov, V. A. Chem. Heterocycl. Compd. 2014, 50, 195. [Khim. Geterotsikl. Soedin. 2014, 219.]
Belikov, M. Yu.; Ievlev, M. Yu.; Belikova, I. V.; Ershov, O. V.; Tafeenko, V. A.; Surazhskaya, M. D. Chem. Heterocycl. Compd. 2015, 51, 518. [Khim. Geterotsikl. Soedin. 2015, 51, 518.]
Krasikovs, A. Chem. Heterocycl. Compd. 2015, 51, 385. [Khim. Geterotsikl. Soedin. 2015, 51, 385.]
Boger, D. L.; Boyce, C. W.; Labrili, M. A.; Sehon, C. A.; Lin, Q. J. Am. Chem. Soc. 1999, 121, 54.
Goncalves, M. S. T. Chem. Rev. 2009, 109, 190.
Estevez, V.; Villacampa, M.; Menendez, J. C. Chem. Soc. Rev. 2010, 39, 4402.
Arthur, R. C.; Gupton, T. J.; Kellogg, E. G.; Yeudall, W. A.; Cabot, C. M.; Newsham, I. F.; Gewirtz, A. D. Biochem. Pharmacol. 2007, 74, 981.
Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213.
Alizadeh, A.; Babaki, M.; Zohreh, N. Tetrahedron 2009, 65, 1704.
Chong, Q.; Xin, X.; Wang, C.; Wu, F.; Wan, B. Tetrahedron 2014, 70, 490.
Lee, H.; Kim, B. Tetrahedron 2013, 69, 6698.
Farahi, M.; Tamaddon, F.; Karami, B.; Pasdar, S. Tetrahedron Lett. 2015, 56, 1887.
(a) Remers, W. A.; Brown, R. K. Indoles, Part One; Houlihan, W. J., Ed.; John Wiley & Sons, Inc.: New York, 1972, p. 385. (b) Sundberg, R. J. The Chemistry of Indoles; Academic Press: New York, 1996.
(a) Casapullo, A.; Bifulco, G.; Bruno, I.; Riccio, R. J. Nat. Prod. 2000, 63, 447. (b) Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C. O.; Sim, C. J.; Im, K. S.; Jung, J. H. J. Nat. Prod. 2005, 68, 711. (c) Kouko, T.; Matsumura, K.; Kawasaki, T. Tetrahedron 2005, 61, 2309. (d) Kaniwa, K.; Arai, M. A.; Li, X.; Ishibashi, M. Bioorg. Med. Chem. Lett. 2007, 17, 4254.
Sabbaghan, M.; Sanaeishoar, H.; Ghalaei, A.; Sofalgar, P. J. Iran. Chem. Soc. 2015, 12, 2199.
Mehrabi, H.; Anary-Abbasinejad, M.; Mirhashemi, F. Tetrahedron Lett. 2014, 55, 4310.
Habibi, D.; Kaamyabi, S.; Hazarkhani, H. Chin. J. Catal. 2015, 36, 362.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(5), 294–298
Rights and permissions
About this article
Cite this article
Babaei, S.E., Hossaini, Z., Besheli, R.R. et al. Fe3O4 nanoparticles as an efficient and reusable catalyst for the solvent-free synthesis of 1H-indole and 1H-pyrrole derivatives. Chem Heterocycl Comp 52, 294–298 (2016). https://doi.org/10.1007/s10593-016-1880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1880-0