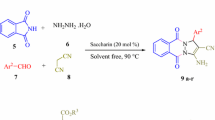
Novel isoxazoline and pyrazoline derivatives of N-substituted saccharin were synthesized in good yields by 1,3-dipolar cycloaddition of N-crotonoyl- or N-cinnamoylsaccharin as dipolarophile to arylnitrile oxides or nitrile imines using p-HAP300 as catalyst under solventfree microwave conditions. In this process, the yields were significantly improved compared to classical conditions without alteration of the selectivity. The regioselectivity as well as the nonthermal specific microwave effect are discussed.




Similar content being viewed by others
References
Jadhav, S. B.; Shastri, R. A.; Gaikwad, K. V.; Gaikwad, S. V. J. Chem. 2009, 6, S183.
Bano, S.; Alam, M. S.; Javed, K.; Mridu Dudeja, M.; Das, A. K.; Dhulap, A. Eur. J. Med. Chem. 2015, 95, 96.
Dianqing Sun, R.; Lee, R. B.; Tangallapally, R. P.; Lee, R. E. Eur. J. Med. Chem. 2009, 44, 460.
Habeeb, A. G.; Rao, P. N. P.; Knaus, E. E. J. Med. Chem. 2001, 44, 2921.
Memeo, M. G.; Lapolla, F.; Giovanni Maga, G.; Quadrelli, P. Tetrahedron Lett. 2015, 56, 1986.
Mondal, P.; Jana, S.; Balaji, A.; Ramakrishna, R.; Kanthal, L. K. J. Young Pharm. 2012, 4, 1.
7.1 Simoni, D.; Giuseppina Grisolia, G.; Giannini, G.; Roberti, M.; Rondanin, R.; Piccagli, L.; Baruchello, R.; Rossi, M.; Romagnoli, R.; Invidiata, F. P.; Grimaudo, S.; Jung, M. K.; Hamel, E.; Gebbia, N.; Crosta, L.; Abbadessa, V.; Di Cristina, A.; Dusonchet, L.; Meli, M.; Tolomeo, M. J. Med. Chem. 2005, 48, 723.
Pinto, D. J. P.; Smallheer, J. M.; Cheney, D. L.; Knabb, R. M.; Wexler, R. R. J. Med. Chem. 2010, 53, 6243.
Klimova, E. I.; Marcos, M.; Klimova, T. B.; Cecilio, A. T.; Ruben, A. T.; Lena, R. R. J. Organomet. Chem. 1999, 585, 106.
Kharbanda, C.; Alam, M. S.; Hamid, H.; Javed, K.; Bano, S.; Dhulap, A.; Ali, Y.; Nazreen, S.; Haider, S. Bioorg. Med. Chem. 2014, 22, 5804.
Palaska, E.; Aytemir, M.; Uzbay, I. T.; Erol, D. Eur. J. Med. Chem. 2001, 36, 539.
Rathore, P.; Yaseen, S.; Ovais, S.; Bashir, R.; Yaseen, R.; Hameed, A. D.; Samim, M.; Gupta, R.; Hussain, F.; Javed, K. Bioorg. Med. Chem. Lett. 2014, 24, 1685.
Sahu, S. K.; Banerjee, M.; Samantray, A.; Behera, A.; Azam, M. A. Trop. J. Pharm. Res. 2008, 7, 961.
Martins, D. M.; Torres, B. G.; Spohr, P. R.; Machado, P.; Bonacorso, H. G.; Zanatta, N.; Martins, M. A.; Emanuelli, T. Basic Clin. Pharmacol. Toxicol. 2009, 104, 107.
Sivakumar, P. M.; Seenivasan, P. S.; Kumar, V.; Doble, M. Bioorg. Med. Chem. Lett. 2010, 20, 3169.
Hassan, S. Y. Molecules 2013, 18, 2683.
Habibullah, K.; Shamshir, K.; Mohamed, J. A.; Bahar, A. Bioorg. Med. Chem. Lett. 2011, 21, 7251.
Kaplancikli, A.; Turan-Zitouni, G.; Ozdemir, A.; Can, O. D.; Chevallet, P. Eur. J. Med. Chem. 2009, 44, 2606.
Zhao, P. L.; Wang, F.; Zhang, M. Z.; Liu, Z. M.; Huang, W.; Yang, G. F. J. Agric. Food Chem. 2008, 56, 10767.
(a) Eftekhari-Sis, B.; Maryam Zirak, M.; Akbari, A. Chem. Rev. 2013, 113, 2958. (b) Appukkuttan, P.; Mehta, V. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2010, 39, 1467.
(a) Nasr El Dine, A.; Khalaf, A.; Grée, D.; Tasseau, O.; Fares, F.; Jaber, N.; Lesot, P.; Hachem, A.; Grée, R. Beilstein J. Org. Chem. 2013, 9, 1943. (b) Fedorova, O. V.; Ovchinnikova, I. G.; Kravchenko, M. A.; Skornyakov, S. N.; Rusinov, G. L.; Chupakhin, O. N.; Charushin, V. N. Chem. Heterocycl. Compd. 2014, 50, 946. [Khim. Geterotsikl. Soedin. 2014, 1027.]
(a) Pinho e Melo, T. M. V. D. Curr. Org. Chem. 2005, 9, 925. (b) Basel, Y.; Hassner, A. Synthesis 1997, 309.
(a) Syassi, B.; Bougrin, K.; Soufiaoui, M. Tetrahedron Lett. 1997, 38, 8855. (b) Ben-Alloum, A.; Bakkas, S.; Bougrin, K.; Soufiaoui, M. New J. Chem. 1998, 22, 809. (c) Bougrin, K.; Loupy, A.; Soufiaoui, M. J. Photochem. Photobiol., C 2005, 6, 139. (d) Mabrour, M.; Bougrin, K.; Benhida, R.; Loupy, A.; Soufiaoui, M. Tetrahedron Lett. 2007, 48, 443. (e) Bougrin, K.; Benhida, R. In Microwaves in Organic Synthesis; De la Hoz, A.; Loupy, A., Eds., 3rd ed.; Wiley-VCH: Weinheim, 2012, Vol. 2, p. 737. (f) Driowya, M.; Puissant, A.; Robert, G.; Auberger, P.; Benhida, R.; Bougrin, K. Ultrason. Sonochem. 2012, 19, 1132. (g) Marzag, H.; Saber, A.; Bougrin, K.; Benhida, R. Curr. Org. Chem. 2014, 18, 2139. (h) Marzag, H.; Robert, G.; Dufies, M.; Bougrin, K.; Auberger, P.; Benhida, R. Ultrason. Sonochem. 2015, 22, 15.
De Monte, C.; Carradori, S.; Secci, D.; D'Ascenzio, M.; Vullo, D.; Ceruso, M.; Supuran, C. T. Eur. J. Med. Chem. 2014, 84, 240.
(a) Blanchet, J.; Macklin, T.; Ang, P.; Metallinos, C.; Snieckus, V. J. Org. Chem. 2007, 72, 3199. (b) Soler, L.; Cerrada, V.; Matia, M. P.; Novella, J. L.; Alvarez-Builla, J. ARKIVOC 2007, (iv), 312.
(a) Loupy, A.; Perreux, L.; Liagre, M.; Burle, K.; Moneuse, M. Pure Appl. Chem. 2001, 3, 161. (b) Perreux, L.; Loupy, A. Tetrahedron 2001, 57, 9199.
Driowya, M.; Bougrin, K.; Benhida, R. In Targets in Heterocyclic Systems – Chemistry and Properties; Attanasi, O. A.; Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, 2011, vol. 15, p. 327.
(a) Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Tetrahedron 2001, 57, 9225. (b) Dallinger, D.; Kappe, C. O. Chem. Rev. 2007, 107, 2563. (c) Microwaves in Organic Synthesis; De la Hoz, A.; Loupy, A., Eds., 3rd ed.; Wiley-VCH: Weinheim, 2012. (d) Cravotto, G.; Tagliapietra, S.; Caporaso, M.; Garella, D.; Borretto, E.; Di Stilo, A. Chem. Heterocycl. Compd. 2013, 49, 811. [Khim. Geterotsikl. Soedin. 2013, 869.]
(a) Ding, J.; Gu, H.; Wen, J.; Lin, C. Synth. Commun. 1994, 24, 301. (b) Fiorino, F.; Caliendo, G.; Perissutti, E.; Severino, B.; Frecentese, F.; Preziosi, B.; Izzo, A. A.; Capasso, R.; Santagada, V. Arch. Pharm. Chem. Life Sci. 2005, 338, 548. (c) Jakopin, Z.; Dolenc M. S. Synth. Commun. 2010, 40, 2464. Ceruso, M.; Supuran, C. T. Bioorg. Med. Chem. 2014, 22, 1821.
(a) Banik, I.; Banik, B. K. Top Heterocycl. Chem. 2013, 30, 183. (b) Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, H. Molecules 2014, 19, 20197.
Zia-ur-Rehman, M.; Choudary J. A.; Ahmad, S. Bull. Korean Chem. Soc. 2005, 26, 1771.
(a) Majumdar, K. C.; Mondal, S. Chem. Rev. 2011, 111, 7749. (b) Gyorgy, K.; Alajos, G.; Erika, B. Curr. Org. Synth. 2013, 10, 751.
Ersanlı, C. C.; Odabaşoğlu, M.; Sarı, U.; Erdönmez, A. Acta Cryst., Sect. C: Struct. Chem. 2005, C61, o243.
(a) Caramella, P.; Reami, D.; Falzoni, M.; Quadrelli, P. Tetrahedron 1999, 55, 7027. (b) Yoshimura, A.; Middleton, K. R.; Todora, A. D.; Kastern, B. J.; Koski, S. R.; Maskaev, A. V.; Zhdankin, V. V. Org. Lett. 2013, 15, 4010.
(a) El-Hammari, L.; Laghzizil, A.; Barboux, P.; Saoiabi, A.; Lahlil, K. J. Solid State Chem. 2004, 177, 134. (b) Mallouk, S.; Bougrin, K.; Laghzizil, A.; Benhida, R. Molecules 2010, 15, 813. 36. (a) Radhakrishna, A. S.; Sivaprakash, K.; Singh, B. B. Synth. Commun. 1991, 21, 1625. (b) Yu, X. -X.; Houk, K. N. J. Am. Chem. Soc. 2003, 125, 13825. (c) Krompiec, S.; Bujak, P.; Malarz, J.; Krompiec, M.; Skórka, L.; Pluta, T.; Danikiewicz, W.; Kania, M.; Kusz, J. Tetrahedron 2012, 68, 6018. (d) Yadav, M. R.; Shirude, S. T.; Puntambekar, D. S.; Patel, P. J.; Prajapati, H. B.; Parmar, A.; Balaraman, R.; Giridhar, R. Acta Pharm. 2007, 57, 13.
(a) Efremova, M. M.; Molchanov, A. P.; Stepakov, A. V.; Kostikov, R. R.; Shcherbakova, V. S.; Ivanov, A. V. Tetrahedron 2015, 71, 2071. (b) Wang, L. J.; Tang, Y. In Comprehensive Organic Synthesis (2nd Ed.); Knochel, P.; Molander, G. A., Eds.; Elsevier: Amsterdam, Vol. 4, p. 1342.
Dirnens, V.; Belyakov, S.; Lukevics, E. Chem. Heterocycl. Compd. 2005, 41, 393. [Khim. Geterotsikl. Soedin. 2005, 450.]
(a) Karmakar, D.; Prajapati, D.; Sandhu, J. S. Synth. Commun. 1998, 28, 2415. (b) Lu, T.-J.; Tzeng, G.-M. J. Chin. Chem. Soc. 2000, 47, 189.
(a) Del Buttero, P.; Molteni, G.; Pilati, T. Tetrahedron 2005, 61, 2413. (b) Abdel-Aziz, H. A.; El-Zahabi, H. S. A.; Dawood, K. M. Eur. J. Med. Chem. 2010, 45, 2427. (c) Chandanshive, J. Z.; Gonzalez, P. B.; Tiznado, W.; Bonini, B. F.; Caballero, J.; Femoni, C.; Franchini, M. C. Tetrahedron 2012, 68, 3319.
Bougrin, K.; Soufiaoui, M.; Loupy, A.; Jacquault, P. New J. Chem. 1995, 19, 21.
Zahouily, M.; Salah, M.; Bahlaouane, B.; Rayadh, A.; Houmam, A.; Hamed, E. A.; Sebtic, S. Tetrahedron 2004, 60, 1631.
SAINT, Bruker AXS, Inc.: Madison, 2007.
SADABS, Bruker AXS, Inc.: Madison, 2001.
Burla, C. M.; Camalli, M.; Carrozzini, B.; Cascarano, G. L.; iacovazzo, C.; Poliori, G.; Spagna, R. J. Appl. Crystallogr. 2003, 36, 1103.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(1), 31–40
Rights and permissions
About this article
Cite this article
Saber, A., Driowya, M., Alaoui, S. et al. Solvent-Free Regioselective Synthesis of Novel Isoxazoline and Pyrazoline N-Substituted Saccharin Derivatives Under Microwave Irradiation. Chem Heterocycl Comp 52, 31–40 (2016). https://doi.org/10.1007/s10593-016-1828-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1828-4