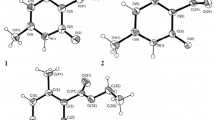
The reduction of quaternary 1-(adamantan-1-yl)pyridinium salts with sodium borohydride in ethanol gave 1-(adamantan-1-yl)-1,2,3,6-tetrahydropyridines that reacted with benzene in trifluoromethanesulfonic acid medium, leading to the formation of 1-(adamantan-1-yl)-phenylpiperidines with various spatial orientation of the phenyl substituent. The structure of the obtained phenylpiperidines was confirmed by the spectral dataset. The thermodynamic stability calculations for the conformers of phenylpiperidines were performed with the B3LYP/6-311++(d,p) method.










Similar content being viewed by others
References
(a) Buffat, M. G. P. Tetrahedron 2004, 60, 1701. (b) Matveeva, N. N.; Winfield, L. L.; Redda, K. K. Curr. Med. Chem. 2005, 12, 551. (c) Fries, D. S.; de Vries, J.; Hazelhoff, B.; Horn, A. S. J. Med. Chem. 1986, 29, 424. (d) Källström, S.; Leino, R. Bioorg. Med. Chem. 2008, 16, 601. (e) Bourin, M.; Chue, P.; Guillon, Y. CNS Drug Rev. 2001, 7, 25. (f) O'Hagan, D. Nat. Prod. Rep. 2000, 5, 435. (g) Pinard, E.; Alberati, D.; Alvarez-Sanchez, R.; Brom, V.; Burner, S.; Fischer, H.; Hauser, N.; Kolczewski, S.; Lengyel, J.; Mory, R.; Saladin, C.; Schulz-Gasch, T.; Stalder, H. ACS Med. Chem. Lett. 2014, 5, 428.
(a) Lee, J.-H.; Seo, S. H.; Lim, E. J.; Cho, N.-C.; Nam, G.; Kang, S. B.; Pae, A. N.; Jeong, N.; Keum, G. Eur. J. Med. Chem. 2014, 74, 246. (b) Russell, M. G. N.; Baker, R.; Billington, D. C.; Knight, A. K.; Middlemiss, D. N.; Noble, A. J. J. Med. Chem. 1992, 35, 2025. (c) Rogers, G. A.; Parsons, S. M.; Anderson, D. C.; Nilsson, L. M.; Bahr, B. A.; Kornreich, W. D.; Kaufman, R.; Jacobs, R. S.; Kirtman, B. J. Med. Chem. 1989, 32, 1217. (d) Gu, X.; Izenwasser, S.; Wade, D.; Housman, A.; Gulasey, G.; Rhoden, J. B.; Savoie, C. D.; Mobley, D. L.; Lomenzo, S. A.; Trudell, M. L. Bioorg. Med. Chem. 2010, 18, 8356. (e) Araki, T.; Mikami, T.; Tanji, H.; Matsubara, M.; Imai, Y.; Mizugaki, M.; Itoyama, Y. Eur. J. Pharm. Sci. 2001, 12, 231. (f) Di Monte, D. A.; Jewell, M. A. In Encyclopedia of the Neurological Science; 2nd ed.; Daroff, R. B., Aminoff, M. J., Eds.; Academic Press: Oxford, 2014, p. 131.
(a) Joubert, J.; Geldenhuys, W. J.; Van der Schyf, C. J.; Oliver, D. W.; Kruger, H. G.; Govender, T.; Malan, S. F. ChemMedChem 2012, 7, 375. (b) Lipton, S. A. Nat. Rev. Drug Discovery 2006, 5, 160. (c) Wanka, L.; Iqbal, K.; Schreiner, P. R. Chem. Rev. 2013, 113, 3516.
(a) Schmidle, C. J.; Mansfield, R. C. J. Med. Chem. Soc. 1955, 77, 5698. (b) Prostakov, N. S.; Varlamov, A. V.; Vasil'ev, G. A. Chem. Heterocycl. Compd. 1977, 13, 639. [Khim. Geterotsikl. Soedin. 1977, 787.] (c) Thompson, D.; Reeves, P. C. J. Heterocycl. Chem. 1983, 20, 771. (d) Conway, R. J.; Valant, C.; Christopoulos, A.; Robertson, A. D.; Capuano B.; Crosby, I. T. Bioorg. Med. Chem. Lett. 2012, 22, 2560. (e) Chen, H.; Liang, X.; Xu, B.; He, X.; Huang, B.; Yuan, M. Molecules 2014, 19, 12048. (f) Anxionnat, B.; Robert, B.; George, P.; Ricci, G.; Perrin, M.-A.; Pardo, D. G.; Cossy, J. J. Org. Chem. 2012, 77, 6087. (e) Sargsyan, M. S.; Hayotsyan, S. S.; Khachatryan, A. Kh.; Badasyan, A. E.; Panosyan, G. A.; Kon'kova, S. G. Chem. Heterocycl. Compd. 2013, 48, 1805. [Khim. Geterotsikl. Soedin. 2012, 1928.]
(a) Klumpp, D. A.; Beauchamp, P. S.; Sanchez, G. V., Jr.; Aguirre, S.; de Leon, S. Tetrahedron Lett. 2001, 42, 5821. (b) Olah, G. A., Klumpp D. A. Superelectrophiles and their Chemistry; Wiley-Intersciense: Hoboken, 2008, p. 250.
Shadrikova, V. A.; Golovin, E. V.; Klimochkin, Y. N. Chem. Heterocycl. Compd. 2015, 50, 1586. [Khim. Geterotsikl. Soedin. 2014, 1725.]
(a) Grierson, D. S.; Harris, M.; Husson, H. J. Am. Chem. Soc. 1980, 102, 1064. (b) Wichitnithad, W.; O'Callaghan, J. P.; Miller, D. B.; Train, B. C.; Callery, P. S. Bioorg. Med. Chem. 2011, 19, 7482. (c) Rouchaud, A.; Kem, W. R. J. Heterocycl. Chem. 2010, 47, 569. (d) Terentiev, P. B.; Zilberstein, T. M.; Borisenko, A. A.; Shmorgunov, V. A.; Piskunkova, N. F.; Grishina, G. V. Chem. Heterocycl. Compd. 2003, 39, 885. [Khim. Geterotsikl. Soedin. 2003, 1027.] (e) Keay, J. G. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon Press: Oxford, 1991, vol. 8, chap. 3.6; p. 579.
(a) Ischay, M. A.; Takase, M. K.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2013, 135, 2478. (b) Teichert, J. F.; Zhang, S.; van Zijl, A. W.; Slaa, J. W.; Minnaard, A. J.; Feringa, B. L. Org. Lett. 2010, 12, 4658.
(a) Eliel, E. L.; Wilen, S. H.; Doyle, M. P. Basic Organic Stereochemistry; Wiley-Interscience; New York, 2001, p. 448. (b) Salamone, M.; Martella, R.; Bietti, M. J. Org. Chem. 2012, 77, 8556.
(a) Klimochkin, Y. N.; Leonova, M. V.; Korzhev, I. R.; Moiseev, I. K.; Vladyko, G. V.; Korobchenko, L. V.; Boreko, E. I.; Nikolaeva, S. N. Pharm. Chem. J. 1992, 26, 616. [Khim.-Farm. Zh., 1992, 26, 58.] (b) Kevill, D. N.; Upadhyay, V. J. Phys. Org. Chem. 1997, 10, 600.
(a) Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2004, 42, 955. (b) Basso, E. A.; Gauze, G. F.; Abraham, R. J. Magn. Reson. Chem. 2007, 45, 749. (c) Rodríguez-Franco, M. I.; Fernández-Bachiller, M. I. Magn. Reson. Chem. 2002, 40, 549. (d) Casy, A. F.; Dewar, G. H.; Al Deeb, O. A. A. Chirality 1989, 1, 202. (e) Casy, A. F.; Ogungbamila, F. O. Org. Magn. Reson. 1982, 18, 171.
(a) Cheng, A.; Uyeno, E.; Polgar, W.; Toll, L.; Lawson, J. A.; DeGraw, J. I.; Loew, G.; Camerman, A.; Camerman, N. J. Med. Chem. 1986, 29, 531. (b) Li, R.-L.; Liu, G.-Q.; Li, W.; Wang, Y.-M.; Li, L.; Duan, L.; Li, Y.-M. Tetrahedron 2013, 69, 5867. (c) Takemiya, A.; Hartwig, J. F. J. Am. Chem. Soc. 2006, 128, 6042.
Casy, A. F.; Dewar, G. H.; Al-Deeb, O. A. A. Magn. Reson. Chem. 1989, 27, 964.
(a) Parker, W.; Riddell. F. G. In Aliphatic, Alicyclic, and Saturated Heterocyclic Chemistry, Vol. 1, pt. III: Five- and Six- Membered Rings; Medium Sized Rings; Bridged and Caged Systems (Carbocyclic and Saturated Heterocyclic); Parker, W., Ed.; The Chemical Society: London, 1973, p. 41. (b) Pines, H. Chemistry of Catalytic Hydrocarbon Conversions; Academic Press: New York, 1981, p. 18.
(a) Kevill, D. N.; Weitl, F. l. J. Am. Chem. Soc. 1968, 90, 6416. (b) Prakash, G. K. S.; Paknia, F.; Mathew, T.; Mloston, G.; Joschek, J. P.; Olah, G. A. Org. Lett. 2011, 13, 4128. (c) Beak, P.; Trancik, R. J. J. Am. Chem. Soc. 1968, 90, 2714.
Parr, R. G.; Szentpály, L.; Liu, S. J. Am. Chem. Soc. 1999,121, 1922.
Peìrez, P.; Toro-Labbeì, A.; Aizman, A.; Contreras, R. J. Org. Chem. 2002, 67, 4747.
Fürst, A.; Plattner, P. A. Helv. Chim. Acta 1949, 32, 275.
Krumkalns, E. V.; Pfeifer, W. J. Med. Chem. 1968, 11, 1103.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, 2009.
Butov, G. M.; Mokhov, V. M. Russ. J. Org. Chem. 2014, 50, 447. [Zh. Org. Khim. 2014, 50, 455.]
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
This work was supported by a grant from the Russian Scientific Fund (project No. 15-13-0084).
Author information
Authors and Affiliations
Corresponding author
Additional information
The Supplementary information file containing the data of 1H and 13C NMR spectra, 1H–13C HMBC, 1H–13C HETCOR, and NOESY two-dimensional NMR experiments for compounds 2a–f, 4a–е, as well as the log files for calculating the energy of conformers 4b–е is available online at http://link.springer.com/journal/10593.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(10), 891–898
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 14913 kb)
Rights and permissions
About this article
Cite this article
Shadrikova, V.A., Golovin, E.V., Shiryaev, V.A. et al. Synthesis of adamantyl-containing phenylpiperidines. Chem Heterocycl Comp 51, 891–898 (2015). https://doi.org/10.1007/s10593-015-1792-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1792-4