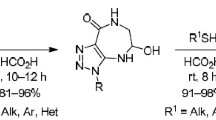
A convenient method was developed for the synthesis of 6-(1H-1,2,3-triazol-1-yl)-4,7-dihydro-1,3,5-dioxazepines, using deoxygenation of 1-(5-nitro-1,3-dioxan-5-yl)-1H-1,2,3-triazoles with triethyl phosphite.


Similar content being viewed by others
References
J. I. G. Cadogan, Synthesis, 11 (1969).
T. Kametani, F. F. Ebetino, T. Yamanaka, and K. Nyu, Heterocycles, 209 (1974).
C. Wentrup, in: A. R. Katritzky (editor), Advances in Heterocyclic Chemistry, Vol. 28, Elsevier, New York (1981), p. 231.
B. C. G. Söderberg, Curr. Org. Chem., 4, 727 (2000).
J. I. G. Cadogan, Acc. Chem. Res., 303 (1972).
G. Smolinsky and B. I. Feuer, J. Org. Chem., 31, 3882 (1966).
T. Kametani, F. F. Ebetino, and K. Fukumoto, Tetrahedron, 30, 2713 (1974).
V. P. Semenov, A. N. Studenikov, and A. A. Potekhin, Chem. Heterocycl. Compd., 14, 233 (1978). [Khim. Geterotsikl. Soedin., 291 (1978).]
V. P. Semenov, A. N. Studenikov, and A. A. Potekhin, Chem. Heterocycl. Compd., 15, 467 (1979). [Khim. Geterotsikl. Soedin., 579 (1979).]
M. R. Devi, J. M. Rao, and V. R. Srinivasan, Synth. Commun., 20, 2301 (1990).
I. V. Ukrainets, L. V. Sidorenko, and O. S. Golovchenko, Chem. Heterocycl. Compd., 43, 1434 (2007). [Khim. Geterotsikl. Soedin., 1687 (2007).]
R. J. Sundberg, B. P. Das, and R. H. Smith, J. Am. Chem. Soc., 91, 658 (1969).
T. de Boer, J. I. G. Cadogan, H. M. McWilliam, and A. G. Rowley, J. Chem. Soc., Perkin Trans. 2, 554 (1975).
S. Trippett and D. M. Walker, J. Chem. Soc., 2976 (1960).
T. Mukaiyama and H. Nambu, J. Org. Chem., 27, 2201 (1962).
P. A. Wehrli and B. Schaer, J. Org. Chem., 42, 3956 (1977).
H. Burgess and J. A. Donnelly, Tetrahedron, 47, 111 (1991).
B. Fischer and L. Sheihet, J. Org. Chem., 63, 393 (1998).
K. S. Kim, E. Y. Hurh, J. N. Youn, and J. I. Park, J. Org. Chem., 64, 9272 (1999).
J. F. Allen, J. Am. Chem. Soc., 79, 3071 (1957).
I. V. Martynov and A. I. Yurtanov, Russ. Chem. Rev., 58, 848 (1989). [Usp. Khim., 1474 (1989).]
M. Ohno and N. Kawabe, Tetrahedron Lett., 7, 3935 (1966).
J. Burdon and A. Ramirez, Tetrahedron, 29, 4195 (1973).
J.-L. Gras, R. Nouguier, and M. Mchich, Tetrahedron Lett., 28, 6601 (1987).
G. B. Linden and M. H. Gold, J. Org. Chem., 21, 1175 (1956).
D. V. Katorov, G. F. Rudakov, and V. F. Zhilin, Russ. Chem. Bull., Int. Ed., 58, 2311 (2009). [Izv. Akad. Nauk, Ser. Khim., 2240 (2009).]
D. V. Katorov, G. F. Rudakov, I. N. Katorova, A. V. Yakushkov, D. P. Simonov, and V. F. Zhilin, Russ. Chem. Bull., Int. Ed., 61, 2114 (2012). [Izv. Akad. Nauk, Ser. Khim., 2098 (2012).]
J. T. Fletcher, S. E. Walz, and M. E. Keeney, Tetrahedron Lett., 49, 7030 (2008).
B. O. Kraiz, Chem. Heterocycl. Compd., 21, 387 (1985). [Khim. Geterotsikl. Soedin., 468 (1985).]
M. Polak and B. Vercek, Synth. Commun., 30, 2863 (2000).
A. Hassner, M. Stern, H. E. Gottlieb, and F. Frolow, J. Org.Chem., 55, 2304 (1990).
P. Magnus, J. Lacour, P. A. Evans, M. B. Roe, and C. Hulme, J. Am. Chem. Soc., 118, 3406 (1996).
K. Niedermann, N. Früh, E. Vinogradova, M. S. Wiehn, A. Moreno, and A. Togni, Angew. Chem., Int. Ed., 50, 1059 (2011).
H.-J. Pi, L.-F. Liu, S.-S. Jiang, W. Du, and W.-P. Deng, Tetrahedron, 66, 6097 (2010).
M. J. Haddadin and A. Hassner, J. Org. Chem., 38, 3466 (1973).
E. Schmidt and R. Wilkendorf, Chem. Ber., 52, 389 (1919).
B. Kedzierski, H. Piotrowska, T. Urbanski, and A. Borys, Rocz. Chem., 46, 1559 (1972).
M. Majewski, D. M. Gleave, and P. Nowak, Can. J. Chem., 73, 1616 (1995).
F. F. Blicke and E. L. Schumann, J. Am. Chem. Soc., 76, 3153 (1954).
Y. Tanaka, S. R. Velen, and S. I. Miller, Tetrahedron, 29, 3271 (1973).
G. M. Sheldrick, Acta Crystallogr., Sec. A: Found. Crystallogr., A64, 112 (2008).
G. M. Sheldrick, SADABS, Program for Scaling and Correction of Area Detector Data, version 2008/1, University of Göttingen, Göttingen (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1777-1790, November, 2014.
Rights and permissions
About this article
Cite this article
Rudakov, G.F., Dubovis, M.V., Kulagin, A.S. et al. Synthesis of Substituted 6-(1H-1,2,3-Triazol-1-Yl)- 4,7-Dihydro-1,3,5-Dioxazepine. Chem Heterocycl Comp 50, 1634–1646 (2015). https://doi.org/10.1007/s10593-014-1633-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1633-x