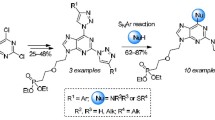
A novel method has been developed for the synthesis of 8-alkyl[1, 2, 4]triazolo[5,1-b]purines from 6-nitro[1,2,4]triazolo[1,5-a]pyrimidin-7-ones via successive phosphoryl chloride-mediated chloro-desoxygenation, aminodehalogenation, and reduction to give 7-alkylamino-6-amino[1,2,4]triazolo-[1,5-a]pyrimidines. The latter can be cyclized in the presence of formic acid.
Similar content being viewed by others
References
E. J. Corey, B. Czakó, and L. Kürti, Molecules and Medicine, John Wiley & Sons, Hoboken (2007), p. 272.
J. Wirsching, J. Voss, J. Balzarini, and E. De Clercq, Bioorg. Med. Chem. Lett., 10, 1339 (2000).
A. Jarrahpour, J. Sheikh, I. El Mounsi, H. Juneja, and T. Ben Hadda, Med. Chem. Res., 22, 1203 (2013).
L. Scagnelli, M. G. Memeo, S. Carosso, B. Bovio, and P. Quadrelli, Eur. J. Org. Chem., 3835 (2013).
D. E. Bergstrom, Unnatural Nucleosides with Unusual Base Pairing Properties. Current Protocols in Nucleic Acid Chemistry, 37:1.4:1.4.1–1.4.32, John Wiley & Sons Inc. (2009). DOI: 10.1002/0471142700.nc0104s37.
R. K. Ujjinamatada, P. Phatak, A. M. Burger, and R. S. Hosmane, J. Med. Chem., 51, 694 (2008).
S. Nakano, M. Fujii, and N. Sugimoto, J. Nucleic Acids, 2011. http://dx.doi.org/10.4061/2011/967098.
C. Burkholder, W. R. Dolbier, Jr., M. Médebielle, and S. Ait-Mohand, Tetrahedron Lett., 42, 3077 (2001).
B. Xu, A. Stephens, G. Kirschenheuter, A. F. Greslin, X. Cheng, J. Sennelo, M. Cattaneo, M. L. Zighetti, A. Chen, S.-A. Kim, H. S. Kim, N. Bischofberger, G. Cook, and K. A. Jacobson, J. Med. Chem., 45, 5694 (2002).
M. Lhassani, O. Chavignon, J.-M. Chezal, J.-C. Teulade, J.-P. Chapat, R. Snoeck, G. Andrei, J. Balzarini, E. De Clercq, and A. Gueiffier, Eur. J. Med. Chem., 34, 271 (1999).
T. Fonseca, B. Gigante, M. M. Marques, T. L. Gilchrist, and E. De Clercq, Bioorg. Med. Chem., 12, 103 (2004).
D. Combs, C. M. Langevine, Y. Qiu, and F. C. Zusi, WO Pat. Appl. 2005011609.
L. Cosyn, K. K. Palaniappan, S.-K. Kim, H. T. Duong, Z.-G. Gao, K. A. Jacobson, and S. Van Calenbergh, J. Med. Chem., 49, 7373 (2006).
L. A. Januar and T. F. Molinski, Org. Lett., 15, 2370 (2013).
D. González-Rodríguez, P. G. A. Janssen, R. Martín-Rapún, I. De Cat, S. De Feyter, A. P. H. J. Schenning, and E. W. Meijer, J. Am. Chem. Soc., 132, 4710 (2010).
B. C. Bookser and N. B. Raffaele, J. Org. Chem., 72, 173 (2007).
J.-Q. Wang, Q.-H. Zheng, X. Fei, X. Liu, T. A. Gardner, C. Kao, S. P. Raikwar, B. E. Glick-Wilson, M. L. Sullivan, B. H. Mock, and G. D. Hutchins, Synth. Commun., 34, 917 (2004).
J.-Q. Wang, Q.-H. Zheng, X. Fei, B. H. Mock, and G. D. Hutchins, Bioorg. Med. Chem. Lett., 13, 3933 (2003).
Y. Fuchi, Y. Koga, O. Nakagawa, and S. Sasaki, Tetrahedron, 67, 6746 (2011).
A. E. A. Hassan, P. Wang, A. K. Watanabe, R. T. McBrayer, J. M. Otto, J. L. Stuyver, M. P. Tharnish, and F. R. Schinazi, Nucleosides, Nucleotides Nucleic Acids, 24, 961 (2005).
N. Bomholt, P. T. Jørgensen, and E. B. Pedersen, Bioorg. Med. Chem. Lett., 21, 7376 (2011).
S. Ikeda, K. Sugizaki, H. Yanagisawa, and A. Okamoto, Org. Biomol. Chem., 9, 4176 (2011).
T. Shiota, T. Yamamori, K. Sakai, M. Kiyokawa, T. Honma, M. Ogawa, K. Hayashi, N. Ishizuka, K. Matsumura, M. Hara, M. Fujimoto, T. Kawabata, and S. Nakajima, Chem. Pharm. Bull., 47, 928 (1999).
U. Hempel, E. Lippmann, and E. Tenor, Z. Chem., 30, 320 (1990).
V. L. Rusinov, M. N. Kushnir, and O. N. Chupakhin, Zh. Org. Khim., 27, 2461 (1991).
V. L. Rusinov, E. N. Ulomsky, O. N. Chupakhin, M. M. Zubairov, A. B. Kapustin, N. I. Mitin, M. I. Zhirovetskii, and I. A. Vinogradov, Khim.-Farm. Zh., 24, No. 9, 41 (1990). [Pharm. Chem. J., 24, 646 (1990)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 953-961, June, 2014.
Rights and permissions
About this article
Cite this article
Savateev, K.V., Ulomsky, E.N., Borisov, S.S. et al. 8-Alkyl[1,2,4]Triazolo[5,1-b]Purines. Chem Heterocycl Comp 50, 880–887 (2014). https://doi.org/10.1007/s10593-014-1542-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1542-z