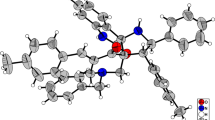
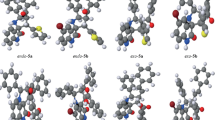
The 1,3-dipolar cycloaddition of the unstabilized azomethine ylides generated in situ from isatin and proline to the endocyclic C = C bond of the tropylidene ring is absolutely regio- and diastereoselective. The local electrophilicity of the dipolarophile and the local nucleophilicity of the azomethine ylide calculated by the B3LYP/6-31G** method are useful tools for predicting the regioselectivity of cycloaddition. The structure and the three-dimensional structure of the synthesized spiropyrrolizidineoxyindoles were established by correlation NMR spectroscopy and X-ray structural analysis.




Similar content being viewed by others
References
M. E. Heitzman, C. C. Neto, E. Winiarz, and A. J. Vaisberg, G. B. Hammond, Phytochemistry, 66, 5 (2005).
C. Marti, and E. M. Carreira, Eur. J. Org. Chem., 2209 (2003).
C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 46, 8748 (2007).
A. S. Girgis, Eur. J. Med. Chem., 44, 91 (2009).
G. Periyasami, R. Raghunathan, G. Surendiran, and N. Mathivanan, Eur. J. Med. Chem., 44, 959 (2009).
A. A. Raj, R. Raghunathan, M. R. Sridevi Kumari, and N. Raman, Bioorg. Med. Chem., 11, 407 (2003).
R. S. Kumar, S. M. Rajesh, S. Perumal, D. Banerjee, P. Yogeeswari, and D. Sriram, Eur. J. Med. Chem., 45, 411 (2010).
M. A. Ali, R. Ismail, T. S. Choon, Y. K. Yoon, A. C. Wei, S. Pandian, R. S. Kumar, H. Osman, and E. Manogaran, Bioorg. Med. Chem. Lett., 20, 7064 (2010).
Y. Zhao, D. Bernard, and S. Wang, BioDiscovery, 8, 4 (2013). DOI: 10.7750/BioDiscovery.2013.8.4.
C. Najera and J. M. Sansano, Curr. Org. Chem., 7, 1105 (2003).
E. P. Olekhnovich, S. L. Boroshko, G. S. Borodkin, I. V. Korobka, V. I. Minkin, and L. P. Olekhnovich, Zh. Org. Khim., 33, 267 (1997). [Russ. J. Org. Chem., 33, 234 (1997).]
T. G. W. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. K. K. N. Vreven, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. G. A. Rega, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. R. A. D. Malick, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. C. M. Nanayakkara, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision E.01, Gaussian, Inc., Wallingford (2004).
R. G. Parr, L. v. Szentpály, and S. Liu, J. Am. Chem. Soc., 121, 1922 (1999).
P. R. Schreiner, L. V. Chernish, P. A. Gunchenko, E. Y. Tikhonchuk, H. Hausmann, M. Serafin, S. Schlecht, J. E. P. Dahl, R. M. K. Carlson, and A. A. Fokin, Nature, 477, 308 (2011).
Houben Weyl, Methods of Organic Chemistry, Vol. II, Methods of Analysis [Russian translation], Goskhimizdat, Moscow (1963), pp. 109–180.
E. P. Olekhnovich, S. L. Boroshko, G. S. Borodkin, V. I. Minkin, and L. P. Olekhnovich, Zh. Org. Khim., 32, 891 (1996). [Russ. J. Org. Chem., 32, 859 (1996).]
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 64A, 112 (2008).
A. V. Tkachuk, S. V. Kurbatov, O. N. Burov, M. E. Kletskii, Yu. P. Tavunova, P. G. Morozov, V. A. Voronina, and V. I. Minkin, Zh. Org. Khim., 49, 1388 (2013). [Russ. J. Org. Chem., 49, 1373 (2013).]
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 32-40, January, 2014.
Rights and permissions
About this article
Cite this article
Tkachuk, A.V., Kurbatov, S.V., Burov, O.N. et al. Synthesis of Spirocyclic Pyrrolizidineoxyindoles with a Furotropylidene Fragment by 1,3-Dipolar Cycloaddition. Chem Heterocycl Comp 50, 26–34 (2014). https://doi.org/10.1007/s10593-014-1444-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1444-0