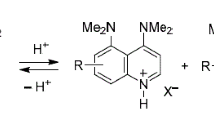
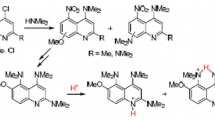
A study on the synthesis of derivatives of 4,5-bis(dimethylamino)quinoline, which is a quinoline analog of 1,8-bis(dimethylamino)naphthalene (also known by its trade name Proton Sponge) was carried out. The first two representatives of this series were obtained. Depending on the aggregate state, solvent, and structural features, these compounds may be protonated either at the quinoline heteroatom or peri-NMe2 groups.
Similar content being viewed by others
References
A. F. Pozharskii, V. A. Ozeryanskii, and E. A. Filatova, Khim. Geterotsikl. Soedin., 208 (2012). [Chem. Heterocycl. Compd., 48, 200 (2012)].
T. Ishikawa (editor), Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts, Wiley, Chichester (2009).
R. W. Alder, P. S. Bowman, W. R. S. Steele, and D. R. Winterman, Chem. Commun. (London), 723 (1968).
A. F. Pozharskii and V. A. Ozeryanskii, in: Z. Rappoport (editor), The Chemistry of Anilines, Part 2, Wiley, Chichester (2007), p. 931.
F. Hibbert, J. Chem. Soc., Perkin Trans. 2, 1862 (1974).
R. L. Benoit, D. Lefebvre, and M. Fréchette, Can. J. Chem., 65, 996 (1987).
I. Kaljurand, A. Kütt, L. Sooväli, T. Rodima, V. Mäemets, I. Leito, and I. A. Koppel, J. Org. Chem., 70, 1019 (2005).
M. A. Zirnstein and H. A. Staab, Angew. Chem., Int. Ed., 26, 460 (1987).
H.-U. Wüstefeld, W. C. Kaska, F. Schüth, G. D. Stucky, X. Bu, and B. Krebs, Angew. Chem., Int. Ed., 40, 3182 (2001).
R. Schwesinger, M. Mißfeldt, K. Peters, and H. G. von Schnering, Angew. Chem., Int. Ed., 26, 1165 (1987).
F. B. Scriven, Chem. Soc. Rev., 12, 129 (1983).
M. R. Heinrich, H. S. Klisa, H. Mayr, W. Steglich, and H. Zipse, Angew. Chem., Int. Ed., 42, 4826 (2003).
A. McCurdy, L. Jimenez, D. A. Stauffer, and D. A. Dougherty, J. Am. Chem. Soc., 114, 10314 (1992).
A. J. Kirby and J. M. Percy, Tetrahedron, 44, 6903 (1988).
J. Verbeek, A. V. E. George, R. L. P. de Jong, and L. Brandsma, J. Chem. Soc., Chem. Commun., 257 (1984).
Y. Kondo, M. Shilai, M. Uchiyama, and T. Sakamoto, J. Am. Chem. Soc., 121, 3539 (1999).
B. Klei and J. H. Teuben, J. Chem. Soc., Chem. Commun., 659 (1978).
F. Mongin and G. Quéguiner, Tetrahedron, 57, 4059 (2001).
D. L. Comins, H. Hong, J. K. Saha, and G. Jianhua, J. Org. Chem., 59, 5120 (1994).
A.-S. Rebstock, F. Mongin, F. Trécourt, and G. Quéguiner, Tetrahedron Lett., 43, 767 (2002).
A. Godard, J.-M. Jacquelin, and G. Quéguiner, J. Organomet. Chem., 354, 273 (1988).
Y. Tagawa, T. Kawaoka, and Y. Goto, J. Heterocycl. Chem., 34, 1677 (1997).
A.-S. Rebstock, F. Mongin, F. Trécourt, and G. Quéguiner, Org. Biomol. Chem., 2, 291 (2004).
C. Kaneko, A. Yamamoto, and M. Gomi, Heterocycles, 12, 227 (1979).
P. D. Woodgate, J. M. Herbert, and W. A. Denny, Heterocycles, 26, 1029 (1987).
J. W. Bunting and W. G. Meathrel, Can. J. Chem., 52, 951 (1974).
E. Ochiai, J. Org. Chem., 18, 534 (1953).
Yu. N. Bubnov, M. E. Gurskii, and T. V. Potapova, Izv. Akad. Nauk, Ser. Khim., 2807 (1996). [Russ. Chem. Bull., 45, 2665 (1996)].
F. W. Vierhapper and E. L. Eliel, J. Org. Chem., 40, 2729 (1982).
M. Hönel and F. W. Vierhapper, J. Chem. Soc., Perkin Trans. 1, 2607 (1982).
M. Hönel and F. W. Vierhapper, Monatsh. Chem., 115, 1219 (1984).
K. A. Skupinska, E. J. McEachern, R. T. Skerlj, and G. J. Bridger, J. Org. Chem., 67, 7890 (2002).
B. Zacharie, N. Moreau, and C. Dockendorff, J. Org. Chem., 66, 5264 (2001).
A. I. Tochilkin, I. R. Kovel’man, E. P. Prokof’ev, I. N. Gracheva, and M. V. Levinskii, Khim. Geterotsikl. Soedin., 1084 (1988). [Chem. Heterocycl. Compd., 24, 8892 (1988)].
R. H. Baker, C. J. Albisetti, R. M. Dodson, G. R. Lappin, and B. Riegel, J. Am. Chem. Soc., 68, 1532 (1946).
R. W. Gouley, G. W. Moersch, and H. S. Mosher, J. Am. Chem. Soc., 69, 303 (1947).
L. A. Ruchelman, J. E. Kerrigan, T.-K. Li, N. Zhou, A. Liu, L. F. Liu, and E. J. LaVoie, Bioorg. Med. Chem., 12, 3731 (2004).
J. C. E. Simpson and P. H. Wright, J. Chem. Soc., 1707 (1948).
A. Adams and D. H. Hey, J. Chem. Soc., 2092 (1950).
W. A. Denny, G. J. Atwell, P. B. Roberta, R. F. Anderson, M. Boyd, C. J. L. Lock, and W. R. Wilson, J. Med. Chem., 35, 4832 (1992).
O. V. Dyablo, E. A. Shmoilova, A. F. Pozharskii, V. A. Ozeryanskii, O. N. Burov, and Z. A. Starikova, Org. Lett., 14, 4134 (2012).
A. F. Pozharskii, E. A. Zvezdina, V. I. Sokolov, and I. S. Kashparov, Chem. Ind. (Chichester, U. K.), 256 (1972).
T. Birchal and W. I. Jolly, J. Am. Chem. Soc., 88, 5439 (1966).
H. A. Staab, A. Kirsch, T. Barth, C. Krieger, and F. A. Neugebauer, Eur. J. Org. Chem., 1617 (2000).
B. Kanner, Heterocycles, 18, 411 (1982).
E. A. Filatova, I. V. Borovlev, A. F. Pozharskii, Z. A. Starikova, and N. V. Vistorobskii, Mendeleev Commun., 10, 178 (2000).
F. Hibbert, Acc. Chem. Res., 17, 115 (1984).
V. A. Ozeryanskii, A. F. Pozharskii, A. Filarowski, and G. S. Borodkin, Org. Lett., 15, 2194 (2013).
J. Azizian, H. Kefayati, M. Mehrdad, K. Jadidi, and Y. Sarrafi, Iran J. Chem. Chem. Eng., 20, 20 (2001).
R. W. Gouley, G. W. Moersch, and H. S. Mosher, J. Am. Chem. Soc., 69, 303 (1947).
M. Karramkam, F. Dolle, H. Valette, L. Besret, Y. Bramoulle, F. Hinnen, F. Vaufrey, C. Franklin, S. Bourg, C. Coulon, M. Ottaviani, M. Delaforge, C. Loch, M. Bottlaender, and C. Crouzela, Bioorg. Med. Chem., 10, 2611 (2002).
E. Ochiai and T. Okamoto, J. Pharm. Soc. Jpn, 68, 88 (1948); Chem. Abstr., 47, 8073 (1953).
This work was carried out with the financial support of the Russian Foundation for Basic Research (grant 11-03-00073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 9, pp. 1404-1419, September, 2013.
Rights and permissions
About this article
Cite this article
Shmoilova, E.A., Dyablo, O.V. & Pozharskii, A.F. Synthesis of 4,5-Bis(Dimethylamino)Quinolines and the Dual Direction of their Protonation. Chem Heterocycl Comp 49, 1308–1322 (2013). https://doi.org/10.1007/s10593-013-1380-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1380-4