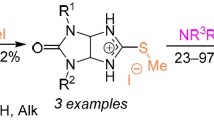
The thermally activated or microwave-induced one-pot three-component condensation of arylglyoxal hydrates, 1,3-dimethylbarbituric acid, and guanidine salts or methylisothiuronium hydroiodide gave respectively 2-amino-5-aryl- and 5-aryl-2-methylsulfanylimidazoles containing a 1,3-dimethylbarbituric acid residue. An unexpected course for the condensation was discovered in the case of guanidine hydrochloride, leading to 5-(2-aryl-2-oxoethyl)-1,3-dimethylbarbituric acids. 2-Amino-2-arylimidazoles under the conditions of such three-component condensation formed Michael adducts involving 5-(2-aryl-2- oxoethylidene)-1,3-dimethylbarbituric acids and the C-5 atom of the imidazole ring.
Similar content being viewed by others
References
F. Bellina, S. Cauteruccio, and R. Rossi, Tetrahedron, 63, 4571 (2007).
D. Sharma, B. Narasimhan, P. Kumar, V. Judge, R. Narang, E. De Clercq, and J. Balzarini, Eur. J. Med. Chem., 44, 2347 (2009).
A. Puratchikodya and M. Doble, Bioorg. Med. Chem. Lett., 15, 1083 (2007).
V. Malhotra, S. R. Pathak, R. Nath, D. Mukherjee, and K. Shanker, Bioorg. Med. Chem. Lett., 21, 936 (2011).
P. Gupta, S. Hameed, and R. Jain, Eur. J. Med. Chem., 39, 805 (2004).
S. D. Sharma, P. Hazarika, and D. Konwar, Tetrahedron Lett., 49, 2216 (2008).
A. Khalafi-Nezhad, M. N. Soltani Rad, G. H. Hakimelahi, and B. Mokhtari, Tetrahedron, 58, 10341 (2002).
Y. Özkay, I. Işikdağ, Z. İncesu, and G. Akalin, Eur. J. Med. Chem., 45, 3320 (2010).
H. M. Refaat, Eur. J. Med. Chem., 45, 2949 (2010).
C. Congiu, M. T. Cocco, and V. Onnis, Bioorg. Med. Chem. Lett., 18, 989 (2008).
F. Hadizadeh, H. Hosseinzadeh, V. S. Motamed-Shariaty, M. Seifi, and S. H. Kazemi, Iran J. Pharm. Res., 7, 29 (2008).
J. A. Jablonowski, K. S. Ly, M. Bogenstaetter, C. A. Dvorak, J. D. Boggs, L. K. Dvorak, B. Lord, K. L. Miller, C. Mazur, S. J. Wilson, T. W. Lovenberg, and N. I. Carruthers, Bioorg. Med. Chem. Lett., 19, 903 (2009).
A. K. Takle, M. J. B. Brown, S. Davies, D. K. Dean, G. Francis, A. Gaiba, A. W. Hird, F. D. King, P. J. Lovell, A. Naylor, A. D. Reith, J. G. Steadman, and D. M. Wilson, Bioorg. Med. Chem. Lett., 16, 378 (2006).
S. Laufer, D. Hauser, T. Stegmiller, C. Bracht, K. Ruff, V. Schattel, W. Albrecht, and P. Koch, Bioorg. Med. Chem. Lett., 20, 6671 (2010).
S. Kantevari, S. V. N. Vuppalapati, D. O. Biradar, and L. Nagarapu, J. Mol. Catal. A: Chem., 266, 109 (2007).
S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti, and K. V. Srinivasan, Tetrahedron, 61, 3539 (2005).
S. D. Sharma, P. Hazarika, and D. Konwar, Tetrahedron Lett., 49, 2216 (2008).
S. N. Murthy, B. Madhav, and Y. V. D. Nageswar, Tetrahedron Lett., 51, 5252 (2010).
K. Ramesh, S. N. Murthy, K. Karnakar, Y. V. D. Nageswar, K. Vijayalakhshmi, B. L. A. Prabhavathi Devi, and R. B. N. Prasad, Tetrahedron Lett., 53, 1126 (2012).
A. Teimouri and A. N. Chermahini, J. Mol. Catal. A: Chem., 346, 39 (2011).
S. Samai, G. C. Nandi, P. Singh, and M. S. Singh, Tetrahedron, 65, 10155 (2009).
N. N. Kolos, L. L. Zamigailo, and V. I. Musatov, Khim. Geterotsikl. Soedin., 1220 (2009). [Chem. Heterocycl. Compd., 45, 970 (2009)].
N. N. Kolos, L. L. Gozalishvili, L. Yu. Kovalenko, and T. V. Berezkina, in: Proceedings of the Twenty- First Ukrainian Conference on Organic Chemistry [in Russian], Cherniga (2007), p. 198.
N. N. Kolos, L. L. Gozalishvili, E. N. Sivokon', and I. V. Knyazeva, Zh. Org. Khim., 45, 124 (2009).
N. N. Kolos, L. L. Zamigailo, N. V. Chechina, I. V. Omel'chenko, O. V. Shishkin, and E. V. Vashchenko, Khim. Geterotsikl. Soedin., 1941 (2012). [Chem. Heterocycl. Compd., 48, 1817 (2012)].
K. Asahi and H. Nishino, Eur. J. Chem., 14, 2404 (2008).
L. L. Gozalishvili, T.V. Beryozkina, I. V. Omelchenko, R. I. Zubatyuk, 0. V. Shishkin, and N. N. Kolos, Tetrahedron, 64, 8759 (2008).
N. N. Kolos, T. B. Beryozkina, and V. D. Orlov, Mendeleev Commun., 12, 91 (2002).
V. V. Lipson, N. V. Svetlichnaya, S. V. Shishkina, and 0. V. Shishkin, Mendeleev Commun., 18, 141 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 935-944, June, 2013.
Rights and permissions
About this article
Cite this article
Kolos, N.N., Chechina, N.V., Zamigailo, L.L. et al. Simple and efficient synthesis of trisubstituted imidazoles. Chem Heterocycl Comp 49, 872–881 (2013). https://doi.org/10.1007/s10593-013-1321-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1321-2