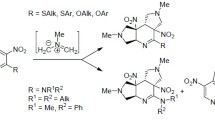
A summary is given of our results and literature data on the use of nitroarenes in pericyclic [4+2] and [3+2] cycloaddition reactions to give heterocycles fused with the original aromatic carbocycle. Highly electrophilic bicyclic nitroarenes, which are considered superelectrophiles, are capable of undergoing the Diels–Alder reaction with nucleophilic alkenes as C=C–N(O)=O heterodienes to give dihydro-1,2-oxazine N-oxides. Dinitrobenzenes, 1,3,5-trinitrobenzene, and their substituted derivatives and analogs as well as nitrobenzenes and 1,3-dinitrobenzenes fused with electron-withdrawing azoles and pyridine are capable of undergoing 1,3-dipolar cycloaddition as dipolarophiles at the C=C(NO2) bond with N-alkylazomethine ylides serving as 1,3-dipoles to give N-alkylpyrrolidines, pyrrolines, or pyrroles fused to an aromatic ring, depending on the structure of the nitro compound substrates. A relationship was found between the reactivity of the nitroarenes in pericyclic [4+2] and [3+2] cycloaddition reactions and the electrophilicity of these substrates.

Similar content being viewed by others
References
I. Fleming, Pericyclic Reactions, Oxford University Press, Oxford (1999).
W. Carruthers, Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford (1990).
S. Kobayashi and K. A. Jorgensen, Cycloaddition Reactions in Organic Synthesis, Wiley, New York (2001).
A. Wasserman, Diels–Alder Reactions, Elsevier, New York (1965).
A. S. Onishchenko, Diene Synthesis [English translation], Israel Programme Translations, Jerusalem (1964).
W. Carruthers, Some Modern Methods of Organic Synthesis, Cambridge University Press, Cambridge (1978).
F. Fringuelli and A. Tatichi, The Diels–Alder Reaction: Selected Practical Methods, Wiley, New York (2001).
W. Oppolzer, Angew. Chem., Int. Ed. Engl., 23, 876 (1984).
K. Narasaka, Synthesis, 1 (1991).
A. Padwa (editor), 1,3-Dipolar Cycloaddition Chemistry, Wiley, New York (1984).
A. Padwa and W. H. Pearson, Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products, Wiley, New York (2002).
R. Huisgen and W. Scheer, Tetrahedron Lett., 12, 481 (1971).
K. V. Gothel and K. A. Jorgensen, Chem. Rev., 98, 863 (1998).
V. A. Tartakovskii and I. E. Chlenov, Zh. Vses. Khim. Obshch. im. D. I. Mendeleeva, 12, 252 (1977).
K. N. Houk, J. Gonzalez, and Y. Li, Acc. Chem. Res., 28, 81 (1995).
M. L. Kuznetsov, Usp. Khim., 75, 1045 (2006). [Russ. Chem. Rev., 75, 935 (2006)].
V. D. Kiselev and A. I. Konovalov, J. Phys. Org. Chem., 22, 466 (2009).
I. Fleming, Frontier Orbitals and Organic Chemical Reactions, J. Wiley & Sons, New York (1976).
P. Perez, L. R. Domingo, M. L. Aurell, and R. Contreras, Tetrahedron, 59, 3117 (2003).
L. R. Domingo, M. L. Aurell, P. Perez, and R. Contreras, Tetrahedron, 58, 4417 (2002).
P. Perez, L. R. Domingo, M. L. Aurell, and R. Contreras, J. Org. Chem., 68, 3884 (2003).
A. Baranski and V. I. Kelarev, Khim. Geterotsikl. Soedin., 435 (1990). [Chem. Heterocycl. Compd., 26, 371 (1990)].
S. S. Novikov, G. A. Shvekhgeimer, V. V. Sevost'yanova, and V. A. Shlyapochnikov, in: Chemistry of Aliphatic and Alicyclic Nitro Compounds [in Russian], Khimiya, Moscow (1974), p. 239.
H. Pellissier, Tetrahedron, 63, 3235 (2007).
C. Najera and J. M. Sansano, Curr. Org. Chem., 7, 1105 (2010).
L. M. Stanley and M. P. Sibi, Chem. Rev., 108, 2887 (2008).
M. Alvarez-Corral, M. Munoz-Dorado, and I. Rodriguez-Garcia, Chem. Rev., 108, 3174 (2008).
G. Pandey, P. Banerjee, and S. R. Gadre, Chem. Rev., 106, 4484 (2006).
M. Ayerbe, A. Arrieta, and F. P. Cossio, J. Org. Chem., 63, 1795 (1998).
A. Padwa, L. Fisera, K. F. Koehler, A. Rodriguez, and G. S. K. Wong, J. Org. Chem., 49, 276 (1984).
R. Huisgen, H. Hauck, H. Seidl, and M. Burger, Chem. Rev., 102, 1117 (1969).
G. A. Shvekhgeimer, Synthesis, 612 (1976).
N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, ch. 8 (2001), p. 231.
S. E. Denmark and A. Thorarensen, Chem. Rev., 96, 137 (1996).
S. E. Denmark, C. J. Cramer, and J. A. Sternberg, Helv. Chim. Acta, 69, 1971 (1986).
S. E. Denmark, C. J. Cramer, and J. A. Sternberg, Tetrahedron Lett., 27, 3693 (1986).
S. E. Denmark, M. S. Dappen, and C. J. Cramer, J. Am. Chem. Soc., 108, 1306 (1986).
H. Uno, K.-I. Goto, N. Watanabe, and H. Suzuki, J. Chem. Soc., Perkin Trans. 1, 289 (1989).
S. E. Denmark, Y.-C. Moon, C. J. Cramer, M. S. Dappen, and C. B. W. Senanayake, Tetrahedron, 46, 7373 (1990).
S. E. Denmark, Y.-C. Moon, and C. B. W. Senanayake, J. Am. Chem. Soc., 112, 311 (1990).
S. E. Denmark, B. S. Kesler, and Y.-C. Moon, J. Org. Chem., 57, 4912 (1992).
S. E. Denmark, A. Stolle, J. A. Dixon, and V. J. Guagnano, J. Am. Chem. Soc., 117, 2100 (1995).
Y. Tohda, N. Yamawaki, H. Matsui, T. Kawashima, M. Ariga, and Y. Mori, Bull. Chem. Soc. Jpn, 61, 461 (1988).
Y. Tohda, K. Tani, N. Yamawaki, M. Ariga, N. Nishiwaki, K. Kotani, and E. Matsumura, Bull. Chem. Soc. Jpn, 66, 1222 (1993).
J.-E. Backvall, U. Karlsson, and R. Chinchilla, Tetrahedron Lett., 32, 5607 (1991).
A. Barco, S. Benetti, G. P. Pollini, G. Spalluto, and V. Zanirato, Tetrahedron Lett., 32, 2517 (1991).
A. Papchikin, P. Agback, J. Plavec, and J. Chattopadhyaya, J. Org. Chem., 58, 2874 (1993).
F. Terrier, Nucleophilic Aromatic Displacement. The Influence of the Nitro Group, VCH, New York (1991).
V. M. Vlasov, Usp. Khim., 764 (2003). [Russ. Chem. Rev., 72, 681 (2003]).
A. F. Pozharskii, Theoretical Basis of Heterocyclic Chemistry [in Russian], Khimiya, Moscow (1985).
F. Terrier, Chem. Rev., 82, 77 (1982).
G. A. Artamkina, M. P. Egorov, and I. P. Beletskaya, Chem. Rev., 82, 427 (1982).
E. Buncel, M. R. Crampton, M. J. Strauss, and F. Terrier, Electron-Deficient Aromatic- and Heteroaromatic-Base Interactions, Elsevier, Amsterdam (1984).
V. V. Perekalin, E. S. Lipina, V. M. Berestovitskaya, and D. A. Efremov, Nitroalkenes: Conjugated Nitro Compounds, Wiley, New York (1994).
F. Terrier, J. M. Dust, and E. Buncel, Tetrahedron, 68, 1829 (2012).
E. Buncel and F. Terrier, Org. Biomol. Chem., 8, 2285 (2010).
S. Lakhdar, G. Berionni, R. Goumont, and F. Terrier, Lett. Org. Chem., 8, 108 (2011).
T. Boubaker, A. P. Chartrousse, F. Terrier, B. Tangour, J. M. Dust, and E. Buncel, J. Chem. Soc., Perkin Trans. 2, 1627 (2002).
R. Goumont, F. Terrier, D. Vichard, S. Lakhdar, J. M. Dust, and E. Buncel, Tetrahedron, 46, 8363 (2005).
H. Mayr, B. Kempf, and A. R. Ofial, Acc. Chem. Res., 36, 66 (2003).
H. Mayr and M. Patz, Angew. Chem., Int. Ed. Engl., 33, 938 (1994).
F. Terrier, S. Lakhdar, T. Boubaker, and R. Goumont, J. Org. Chem., 70, 6242 (2005).
S. Lakhdar, R. Goumont, T. Boubaker, M. Mokhtari, and F. Terrier, Org. Biomol. Chem., 4, 1910 (2006).
S. Lakhdar, R. Goumont, F. Terrier, T. Boubaker, J. M. Dust, and E. Buncel, Org. Biomol. Chem., 5, 1744 (2007).
S. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial, and H. Mayr, J. Org. Chem., 71, 9088 (2006).
P. MacCormack, J. C. Halle, M. J. Pouet, and F. Terrier, J. Org. Chem., 53, 4407 (1988).
J.-C. Halle, D. Vichard, M.-J. Pouet, and F. Terrier, J. Org. Chem., 62, 7178 (1997).
P. Sepulcri, J. C. Halle, R. Goumont, D. Riou, and F. Terrier, J. Org. Chem., 64, 9254 (1999).
D. Vichard, T. Boubaker, F. Terrier, M.-J. Pouet, J. M. Dust, and E. Buncel, Can. J. Chem., 79, 1617 (2001).
A. M. Starosotnikov, M. A. Leontieva, M. A. Bastrakov, A. V. Puchnin, V. V. Kachala, and S. A. Shevelev, Mendeleev Commun., 20, 165 (2010).
S. Kurbatov, R. Goumont, J. Marrot, and F. Terrier, Tetrahedron Lett., 45, 1037 (2004).
D. V. Steglenko, M. E. Kletsky, S. V. Kurbatov, A. V. Tatarov, V. I. Minkin, R. Goumont, and F. Terrier, J. Phys. Org. Chem., 22, 298 (2009).
A. M. Starosotnikov, M. A. Bastrakov, S. A. Shevelev, R. Goumont, and F. Terrier, in: Abstracts of the International Conference on New Directions in Heterocyclic Chemistry, May 3-8, 2009 [in Russian], Kislovodsk (2009), p. S-441.
M. A. Bastrakov, A. M. Starosotnikov, and S. A. Shevelev, in: Abstracts of the 17 th European Symposium on Organic Chemistry, Crete (2011), p. 1.290.
J. S. Splitter and M. Calvin, J. Org. Chem., 20, 1086 (1955).
G. Buchi and D. E. Ayer, J. Am. Chem. Soc., 78, 689 (1956).
J. L. Charlton and P. De Mayo, Can. J. Chem., 46, 1041 (1968).
J. L. Charlton, C. C. Liao, and P. De Mayo, J. Am. Chem. Soc., 93, 2463 (1971).
J. Leitch, Angew. Chem., 88, 416 (1976).
S. Ranganathan, D. Ranganathan, P. V. Ramachandran, M. K. Mahanty, and S. Bamezai, Tetrahedron, 37, 4171 (1981).
K. Okada, Y. Saito, and M. Oda, J. Chem. Soc., Chem. Commun., 1731 (1992).
R. Chandra Boruah, P. Devi, and J. S. Sandhu, J. Heterocycl. Chem., 16, 1555 (1979).
P. Devi and J. S. Sandhu, J. Chem. Soc., Chem. Commun., 990 (1983).
M. A. Bastrakov, A. M. Starosotnikov, S. Yu. Pechenkin, V. V. Kachala, I. V. Glukhov, and S. A. Shevelev, J. Heterocycl. Chem., 47, 893 (2010).
L. S. Konstantinova, M. A. Bastrakov, A. M. Starosotnikov, I. V. Glukhov, K. A. Lysov, O. A. Rakitin, and S. A. Shevelev, Mendeleev Commun., 20, 353 (2010).
A. M. Starosotnikov, M. A. Bastrakov, S. Yu. Pechenkin, M. A. Leontieva, V. V. Kachala, and S. A. Shevelev, J. Heterocycl. Chem., 48, 824 (2011).
S. Yu. Pechenkin, A. M. Starosotnikov, M. A. Bastrakov, I. V. Glukhov, and S. A. Shevelev, Izv. Akad. Nauk, Ser. Khim., 73 (2012).
A. M. Starosotnikov, D. V. Khakimov, M. A. Bastrakov, S. Yu. Pechenkin, S. A. Shevelev, and T. S. Pivina, Khim. Geterotsikl. Soedin., 271 (2011). [Chem. Heterocycl. Compd., 47, 215 (2011)].
S. Lee, I. Chataigner, and S. R. Piettre, Angew. Chem., Int. Ed., 50, 472 (2011).
A. M. Starosotnikov, M. A. Bastrakov, V. V. Kachala, P. A. Belyakov, I. V. Fedyanin, and S. A. Shevelev, Synlett, 2400 (2012).
S. Yu. Pechenkin, A. M. Starosotnikov, M. A. Bastrakov, V. V. Kachala, and S. A. Shevelev, Mendeleev Commun., 22, 35 (2012).
M. Avalos, R. Babiano, A. Cabanillas, P. Cintas, J. L. Jimenez, and J. C. Palacios, J. Org. Chem., 61, 7291 (1996).
G. W. Gribble, E. T. Pelkey, W. M. Simon, and H. A. Trujillo, Tetrahedron, 56, 10133 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 102-128, January, 2013.
Rights and permissions
About this article
Cite this article
Shevelev, S.A., Starosotnikov, A.M. Pericyclic [4+2] and [3+2] Cycloaddition Reactions of Nitroarenes in Heterocyclic Synthesis. Chem Heterocycl Comp 49, 92–115 (2013). https://doi.org/10.1007/s10593-013-1233-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-013-1233-1