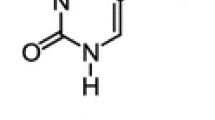
Synthesis, X-ray, and cytotoxicity studies of (S)- and (R)-aziridine-2-carboxamide (Leakadine) are described. X-ray data for the enantiomerically pure form are compared with those for racemic aziridine-2-carboxamide in order to explain the 21°C large melting point difference between both series. It was found that despite their overall low cytotoxicity (S)-aziridine-2-carboxamide is slightly more cytotoxic than (R)-aziridine-2-carboxamide.






Similar content being viewed by others
References
F. M. D. Ismail, D. O. Levitsky, and V. M. Dembitsky, Eur. J. Med. Chem., 44, 3373 (2009).
J. B. Sweeney, Chem. Soc. Rev., 31, 247 (2002).
Y. Nakao, M. Fujita, K. Warabi, S. Matsunaga, and N. Fusetani, J. Am. Chem. Soc., 122, 10462 (2000).
R. S. Coleman and J. Li, A. Navarro, Angew. Chem., Int. Ed., 40, 1736 (2001).
B. S. Iyengar, R. T. Dorr, and W. A. Remers, J. Med. Chem., 47, 218 (2004).
B. S. Iyengar, R. T. Dorr, D. S. Alberts, E. M. Hersh, S. E. Salmon, and W. A. Remers, J. Med. Chem., 42, 510 (1999).
I. Ya. Kalvinsh and E. B. Astapenok, Belg. Pat. Appl. 860239; Chem. Abstr., 90, 34103 (1979).
I. Y. Kalvinsh and E. B. Astapenok, US Pat. Appl. 4686215; Chem. Abstr., 107, 168799 (1987).
P. Trapencieris, I. Kalviņš, L. Kauliņa, and V. Kauss, Org. Process Res. Dev., 1, 259 (1997).
P. J. A. Kuehl and W. A. Remers, WO Pat. Appl. 2008045686; Chem. Abstr., 148, 456463 (2008).
A. Fürst, E. Kyburz, and S. Majnoni, Swiss Pat. Appl. 362078; Chem. Abstr., 59, 62128 (1963).
K. Nakajima, F. Takai, T. Tanaka, and K. Okawa, Bull. Chem. Soc. Jpn., 51, 1577 (1978).
K. Jähnisch, E. Gründemann, A. Kunath, and M. Ramm, Liebigs Ann. Chem., 881 (1994).
J. E. Baldwin, A. C. Spivey, C. J. Schofield, and J. B. Sweeney, Tetrahedron, 49, 6309 (1993).
H. Harada, T. Morie, T. Suzuki, T. Yoshida, and S. Kato, Tetrahedron, 54, 10671 (1998).
G. E. Veitch, K. L. Bridgwood, and S. V. Ley, Org. Lett., 10, 3623 (2008).
M. Turks, D. Zicāne, and I. Rijkure, LV Pat. Appl. 13848; Chem. Abstr., 151, 490989 (2009).
P. T. Trapentsier, I. Ya. Kalvin’sh, E. E. Liepin’sh, and E. Ya. Lukevits, Khim. Geterotsikl. Soedin., 350 (1983). [Chem. Heterocycl. Compd., 19, 283 (1983)].
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
A. A. Starchenko, V. A. Khil'ko, S. A. Komarets, A. N. Khlunovskii, T. I. Prilukova, S. V. Kraskovskaia, and A. V. Danilov, Zh. Vopr. Neirokhir. im. N. N. Burdenko, 15 (1996).
H. E. Gottlieb, V. Kotlyar, and A. Nudelman, J. Org. Chem., 62, 7512 (1997).
A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and R. Spagna, J. Appl. Cryst., 32, 115 (1999).
G. M. Sheldrick, SHELXL-97. Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen, 1997.
S. Mackay, C. J. Gilmore, C. Edwards, N. Stewart, and K. Shankland, maXus. Computer Program for the Solution and Refinement of Crystal Structures. Bruker Nonius, The Netherlands, MacScience, Japan & The University of Glasgow, 1999.
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Professor Ivars Kalvinsh on the occasion of his 65th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 928-935, May, 2012.
Rights and permissions
About this article
Cite this article
Turks, M., Rijkure, I., Belyakov, S. et al. On differences between racemic and enantiomerically pure forms of aziridine-2-carboxamide*. Chem Heterocycl Comp 48, 861–868 (2012). https://doi.org/10.1007/s10593-012-1067-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-1067-2