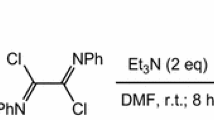
The cyclocondensation of N-(prop-2-yn-1-yl)-o-phenylenediamines with phenyl isothiocyanate leads to the formation of 1-(prop-2-yn-1-yl)-1,3-dihydro-2H-benzimidazole-2-thiones irrespective of the substituent nature at the triple bond. Reactions of both mono- and diacetylenic derivatives of o-phenylenediamine with carbon disulfide in the presence of KOH proceed with the formation of two heterocyclic nuclei simultaneously. From N-(prop-2-yn-1-yl)-o-phenylenediamines containing an aryl substituent at the triple bond, and N-(penta-2,4-diyn-1-yl)-o-phenylenediamines 2-methylidene-2,3-dihydro[1,3]thiazolo[3,2-a]benzimidazoles are formed. The latter are readily isomerized under the action of base giving thiazolo[3,2-a]benzimidazoles. The cyclocondensation of N-(alk-2-yn-1-yl)-o-phenylenediamines with CS2 leads to [1,3]thiazino[3,2-a]benzimidazoles.
Similar content being viewed by others
References
R. Crossley, US Pat. 4873237 (1989); Chem. Abstr., 112, 198401 (1990).
N. H. Grant and D. E. Clark, US Pat. 4361574 (1982); Chem. Abstr., 98, 101200 (1983).
C. M. Rogers, T. J. Rogers, and S. C. Gilman, J. Immunopharmacol., 7, 479 (1985).
R. I. Fenichel, F. J. Gregory, and H. E. Alburn, Br. J. Cancer, 33, 329 (1976).
H. A. Abdel-Aziz, A. M. Gamal-Eldeen, N. A. Hamdy, and I. M. I. Fakhr, Arch. Pharm., 342, 230 (2009).
V. M. Dianov, Khim.-farm. Zh., 41, No. 6, 20 (2007).
J. J. D’Amico, R. H. Campbell, and E. C. Guinn, J. Org. Chem., 29, 865 (1964).
A. E. Alper and A. Taurins, Can. J. Chem., 45, 2903 (1967).
A. N. Krasovskii and P. M. Kochergin, Khim. Geterotsikl. Soedin., 899 (1967). [Chem. Heterocycl. Comp., 3, 709 (1967)].
A. N. Krasovskii, P. M. Kochergin, and L. V. Samoilenko, Khim. Geterotsikl. Soedin., 827 (1970). [Chem. Heterocycl. Comp., 6, 766 (1970)].
A. A. O. Sarhan, H. A. H. El-Sherief, and A. M. Mahmoud, Tetrahedron, 52, 10485 (1966).
K. K. Balasubramanian and B. Venugopalan, Tetrahedron Lett., 15, 2643 (1974).
K. K. Balasubramanian and B. Venugopalan, Tetrahedron Lett., 15, 2645 (1974).
M. M. Heravi, A. Keivanloo, M. Rahimizadeh, M. Bakavoli, and M. Ghassemzadeh, Tetrahedron Lett., 45, 5747 (2004).
A. N. Krasovskii and P. M. Kochergin, Khim.-farm. Zh., 2, No. 10, 18 (1968).
A. A. Shklyarenko, V. V. Yakovlev, and V. N. Chistokletov, Zh. Org. Khim., 40, 617 (2004).
K. Ikeda, S.-I. Hata, Y. Tanaka, and T. Yamamoto, Org. Prep. Proc. Int., 32, 401 (2000).
R. V. Novikov, M. E. Borovitov, and I. A. Balova, Khim. Geterotsikl. Soedin., 627 (2008). [Chem. Heterocycl. Comp., 44, 494 (2008)].
N. Yu. Sipkina and I. A. Balova, Zh. Obshch. Khim., 73, 2002 (2003).
U. Vögeli, W. von Philipsborn, K. Nagarajan, and M. D. Nair, Helv. Chem. Acta, 61, 607 (1978).
P. Sohar, Z. Szöke-Molnar, G. Stajer, and G. Bernath, Magn. Reson. Chem., 27, 959 (1989).
Y. Kurasawa, R. Katoh, A. Takada, H. S. Kim, and Y. Okamoto, J. Heterocycl. Chem., 29, 1001 (1992).
V. S. Berseneva, A. V. Tkachev, Yu. Yu. Morzherin, W. Dehaen, I. Luyten, S. Toppet, and V. A. Bakulev, J. Chem. Soc., Perkin Trans. 1, 2133 (1998).
G. Stájer, A. E. Szabó, and P. Sohár, Heterocycles, 51, 1849 (1999).
V. S. Berseneva, Yu. Yu. Morzherin, W. Dehaen, I. Luyten, and V. A. Bakulev, Tetrahedron, 57, 2179 (2001).
V. A. Bakulev, V. S. Berseneva, N. P. Belskaia, Yu. Yu. Morzherin, A. Zaitsev, W. Dehaen, I. Luyten, and S. Toppet, Org. Biomol. Chem., 1, 134 (2003).
U. Vögeli and W. von Philipsborn, Org. Magn. Reson., 7, 617 (1975).
U. Vögeli, D. Herz, and W. von Philipsborn, Org. Magn, Reson. 13, 200 (1980).
L. Brandsma and H. D. Verkruijsse, Synthesis of Acetylenes, Allenes, and Cumulenes. A Laboratory Manual, Elsevier (1981), p. 221.
The authors are deeply grateful to associate professor S. I. Selivanova for recording the NMR spectra. The authors thank the Saint-Petersburg State University for financial support (Research grant 12.38.14.2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 917–927, June, 2011.
Rights and permissions
About this article
Cite this article
Novikov, R.V., Danilkina, N.A. & Balova, I.A. Cyclocondensation of n-(prop-2-yn-1-yl)- and n-(penta-2,4-diyn-1-yl)- o-phenylenediamines with phenyl isothiocyanate and carbon disulfide. Chem Heterocycl Comp 47, 758–766 (2011). https://doi.org/10.1007/s10593-011-0831-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0831-z