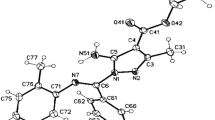
The N-arylation of a series of nitroazoles has been studied with the aid of diaryliodonium salts in the presence of CuI under the action of microwave radiation. It was found that alkylation proceeds regioselectively in each actual case with the formation of one of two possible isomers. The correct structure of the N-arylation products was established on the basis of NMR spectroscopy.



Similar content being viewed by others
References
J. H. Boyer, Nitroazoles, Wiley-VCH, Hamburg (1987), Chaps. 1–5 (1987).
N. G. Huilgol, C. K. K. Nair, and V. T. Kagiya, in: Radiation Sensitizers: A Contemporary Audit, Narosa, Mumbai (2001), Chaps. 1–3.
J. P. Agrawal and R. Hodgson, in: Organic Chemistry of Explosives, Wiley, Chichester (2007), Chaps 1–6 (2007).
M. J. Graneto, US Patent 5521207; Chem. Abs., 125, 114612 (1996).
K. Walczak, A. Gondela, and J. Suwinski, Eur. J. Med. Chem., 39, 849 (2004).
R. Jedrysiak, M. Sawicki, P. Wagner, and J. Suwinski, ARKIVOC, vi, 103 (2007).
M. R. Grimmett, K. H. R. Lim, and R. T. Weavers, Aust. J. Chem., 32, 2203 (1979).
V. A. Chauzov, V. Z. Parchinskii, E. V. Sinel’shchikova, A. V. Burasov, B. I. Ugrak, N. N. Parfenov, and V. A. Petrosyan, Izv. Akad. Nauk, Ser. Khim., 1402 (2002).
A. R. Muci and S. L. Buchwald, Top. Curr. Chem., 219, 131 (2002).
S. V. Ley and A. W. Thomas, Angew. Chem. Intern. Edit. Eng., 42, 5400 (2003).
P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 248, 2337 (2004).
L. Wang and Z.-C. Chen, J. Chem. Res. (S), 367 (2000).
I. P. Beletskaya, D. V. Davydov, and M. Moreno-Manas, Tetrahedron Lett., 39, 5621 (1998).
D. V. Davydov, I. P. Beletskaya, and M. S. Gorovoy, Tetrahedron Lett., 43, 6221 (2002).
D. V. Davydov, I. P. Beletskaya, B. B. Semenov, and Y. A. Smushkevich, Tetrahedron Lett., 43, 6217 (2002).
J. Catalan, J. L. M. Abboud, and J. Elguero, Adv. Heterocycl. Chem., 41, 187 (1987).
V. A. Chertkov and M. A. Yurovskaya, Khim. Geterotsikl. Soedin., 899 (1993). [Chem. Heterocycl. Comp., 29, 762 (1993)].
V. A. Chertkov and N. M. Sergeyev, J. Magn. Reson., 21, 159 (1976).
L. Ernst, V. Wray, V. A. Chertkov, and N. M. Sergeyev, J. Magn. Reson., 25, 123 (1977).
H. Mo and T. C. Pochapsky, Prog. Nucl. Magn. Reson. Spectrosc., 30, 1 (1997).
M. A. Yurovskaya, V. A. Chertkov, A. Z. Afanas'ev, F. V. Ienkina, and Yu. G. Bundel', Khim. Geterotsikl. Soedin., 509 (1985). [Chem. Heterocycl. Comp., 21, 424 (1985)].
M. A. Yurovskaya, A. Z. Afanas’'ev, V. A. Chertkov, A. M. Gizatullina, and Yu. G. Bundel’, Khim. Geterotsikl. Soedin., 1625 (1987). [Chem. Heterocycl. Comp., 23, 1305 (1987)].
M. A. Yurovskaya, A. Z. Afanas'ev, V. A. Chertkov, and Yu. G. Bundel', Khim. Geterotsikl. Soedin., 1213 (1988). [Chem. Heterocycl. Comp., 24, 1000 (1988)].
V. A. Chertkov, Yu. K. Grishin, and N. M. Sergeyev, J. Magn. Reson., 24, 275 (1976).
T. Bundgaard, H. J. Jakobsen, and E. J. Rahkamaa, J. Magn. Reson., 19, 345 (1975).
M. Hansen, R. S. Hansen, and H. J. Jakobsen, J. Magn. Reson., 13, 386 (1974).
V. A. Chertkov and Yu. K. Grishin, Zh. Struk. Khim., 18, 616 (1977).
O. V. Dorofeeva, A. V. Ferenets, N. M. Karasev, L. V. Vilkov, and H. Oberhammer, J. Phys. Chem., A, 112, 5002 (2008).
N. M. Sergeev and V. A. Chertkov, Dokl. Akad. Nauk, 286, 1186 (1986).
W. R. Croasmun and R. M. K. Carlson, Two-dimensional NMR Spectroscopy. Applications for Chemists and Biochemists, Methods in Stereochemical Analysis, Vol. 9, Verlag Chemie, Weinheim (1987).
T. D. W. Claridge, High-Resolution NMR Techniques in Organic Chemistry, 2nd ed., Elsevier, Oxford (2008).
J. A. Jones, Prog. Nucl. Magn. Reson. Spectrosc., 38, 325 (2001).
T. A. Keith and R. F. W. Bader, Chem. Phys. Lett., 210, 223 (1993).
J. R. Cheeseman, M. J. Frisch, G. W. Trucks, and T. A. Keith, J. Chem. Phys., 104, 5497 (1996).
A. K. Shestakova, A. V. Makarkina, O. V. Smirnova, M. M. Shtern, and V. A. Chertkov, Izv. Akad. Nauk, Ser. Khim., 1309 (2006).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09W, Revision A.1, Gaussian Inc., Wallingford (2009).
E. Salwinska and J. Suwinski, Pol. J. Chem., 64, 813 (1990).
J. Suwiski, W. Pawlus, E. Salwiska, and K. Swierczek, Heterocycles, 37, 1511 (1994).
D. Dal Monte Casoni, Gazz. Chim. Ital., 89, 1539 (1959).
M. R. Grimmett, S. R. Hartshorn, K. Schofield, and J. B. Weston, J. Chem. Soc., Perkin Trans. 2, 1654 (1972).
G. S. Predvoditeleva, T. V. Kartseva, and M. N. Shchukina, Khim.-farm. Zh., 8, 525 (1974).
V. A. Petrosyan, M. E. Niyazymbetov, M. S. Pevzner, and B. I. Ugrak, Izv. Akad. Nauk SSSR. Ser. Khim., 1643 (1988).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 63–74, January, 2011.
Rights and permissions
About this article
Cite this article
Chertkov, V.A., Shestakova, A.K. & Davydov, D.V. Regioselective N-arylation of nitroazoles. Determination of the structure of N-arylnitro-azoles on the basis of NMR spectroscopic data and quantum-chemical calculations. Chem Heterocycl Comp 47, 45–54 (2011). https://doi.org/10.1007/s10593-011-0718-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0718-z