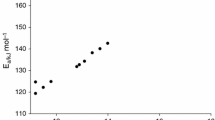
The thermal decomposition study of 3,3,6,6-tetramethyl-1,2,4,5-tetroxane (acetone cyclic diperoxide) was carried out in 2-methoxyethanol solution in the 130-166 °C temperature range. The overall reaction follows a first-order kinetic law up to at least 75% diperoxide conversion. The activation parameters (ΔH# = 22.5 ± 0.7 kcal⋅mol–1 and ΔS# = -25.6 ± 0.5 cal⋅mol–1⋅K–1) for the unimolecular rupture of the O–O bond in the diperoxide molecule were obtained by measuring the remnant diperoxide at different reaction times by the CG technique. Acetone was detected by GC as the major organic product of the reaction.
Similar content being viewed by others
References
J. Kroschwitz (editor), Kirk-Othmer Concise Encyclopedia of Chemical Technology, 4th ed., Organic Peroxides, Wiley, New York, Chichester, 1999, p. 1472.
S. M. Kaye (editor), Encyclopedia of Explosives and Related Items, PATR2700, Vol. 8, US Army Armament Research & Development Command, Dover, NJ, 1978, p. 203.
D. N. S. Hon, Pulp Pap. Can., 86, No. 6, 129 (1985).
T. Yoshida, K. Muraaga, T. Matsunaga, and M. Tamura, J. Hazard. Mater., 12, 27 (1985).
T. Urbanski, Chemistry and Technology of Explosives, Pergamon Press, Oxford, 1967, Vol. 3, p. 299.
F. Dubnikova, R. Kosloff, J. Almong, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt, and E. Keinan, J. Am. Chem. Soc., 127, 1146 (2005).
L. A. C. Leiva, G. B. Castellanos, N. L. Jorge, L. F. R. Cafferata, and M. E. Gómez Vara, Rev. Soc. Quím. Méx., 42, 223 (1998).
A. H. Jubert, R. Pies Diez, and L. F. R. Cafferata, J. Raman Spectr., 30, 479 (1999).
J. A. Y. Riddick and W. B. Bunger, in: A. Weissberger (editor), Organic Solvents, Vol 2, Wiley Intersci., N.Y., 1970.
P.P. Perrin and W. L. F. Armarego, Purification of Laboratory Chemicals, Pergamon Press, 3rd ed., Oxford, 1988.
S. Huyberechts, A. Halleux, and P. Kruys, Bull. Soc. Chim. Belg., 64, 203 (1955).
L. F. R. Cafferata, G. N. Eyler, E. L. Svartman, A. I. Cañizo, and E. E. Alvarez, J. Org. Chem. 56, 411 (1991).
L. F. R. Cafferata, G. N. Eyler, E. L. Svartman, A. I. Cañizo, and E. J. Borkowski, J. Org. Chem., 55, 1058 (1990).
A. I. Cañizo, N. E. Eyler, C. M. Mateo, E. E. Alvarez, and R. K. Nesprias, Heterocycles, 63, 2231 (2004).
B. N. Moryganov, A. I. Kalinin, and L. N. Mikhotova, J. Gen. Chem. (USSR), 32, 3414 (1962).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1806-1811, December, 2009.
Rights and permissions
About this article
Cite this article
Leiva, L.A.C., Romero, J.M., Jorge, N.L. et al. Thermal decomposition reaction of 3,3,6,6-tetramethyl- 1,2,4,5-tetroxane in 2-methoxy- ethanol solution. Chem Heterocycl Comp 45, 1455–1459 (2009). https://doi.org/10.1007/s10593-010-0449-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-010-0449-6