Abstract
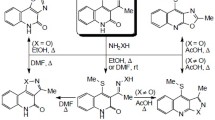
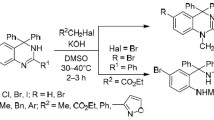
It was shown that 6-acyl-5,13-dihydro-11H-isoquino[3,2-b]quinazolin-11-ones are formed when 5,13-dihydro-11H-isoquino[3,2-b]quinazolin-11-one is heated with the chlorides and anhydrides of carboxylic acids in the presence of bases (pyridine, NaOAc) while 5-acyl-5,13-dihydro-11H-isoquino[3,2-b]quinazolin-11-ones are formed in the presence of NaH. In the presence of NaH 6-acyl-5,13-dihydro-11H-isoquino[3,2-b]quinazolin-11-ones form the products from acylation and alkylation at position 5. The action of heat on 5,13-dihydro-11H-isoquino[3,2-b]quinazolin-11-one in oxalyl chloride leads to 7H,8H-2a,7a-diazacyclopenta[fg]naphthacene-1,2,8-trione.
Similar content being viewed by others
References
L. M. Potikha, V. M. Kisil, A. V. Turov, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 428 (2008). [Chem. Heterocycl. Comp., 44, 330 (2008)].
MDDR [Medline Drug Data Report]; www.discoverygate.com
H. Natsugari, H. Shirafudji, and T. Doi, EP Pat. 566069 Chem. Abstr., 120, 134310 (1994).
M. Fujio et al., WO Pat. 0448339 Chem. Abstr., 141, 385361 (2005).
E. Schefczik, Liebigs Ann. Chem., 729, 83 (1969).
W. Wendelin, H. Keimelmayr, and M. Huber, Sci. Pharm., 56, 437 (1973).
L. M. Potikha, R. M. Gutsul, A. V. Turov, and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 273 (2008). [Chem. Heterocycl. Comp., 44, 208 (2008)].
K. Nagarajan, V. R. Rao, and R. K. Shah, Indian J. Chem., 71, 77 (1988).
M. Bollini, S. E. Asis, and A. M. Bruno, Synthesis, 237 (2006).
L. M. Potikha, V. M. Kisel’, N. V. Danileiko, and V. A. Kovtunenko, 715 (2004). [Chem. Heterocycl. Comp., 40, 609 (2004)].
W. E. Stewart and T. H. Siddal, Chem. Rev., 70, 517 (1970).
A. R. Fersht, J. Am. Chem. Soc., 92, 5432 (1970).
L. M. Potikha and V. A. Kovtunenko, Khim. Geterotsikl. Soedin., 1509 (2007). [Chem. Heterocycl. Comp., 43, 1280 (2007)].
D. A. Filimonov, V. V. Poroikov, Yu. V. Borodina, and T. Gloriozova, J. Chem. Inf. Comput. Sci., 39, 666 (1999).
V. V. Poroikov, D. A. Filimonov, Yu. V. Borodina, A. A. Lagunin, and A. Kos, J. Chem. Inf. Comput. Sci., 40, 1349 (2000).
V. V. Poroikov and D. A. Filimonov, J. Computer-Aided Mol. Design, 16, 819 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 741–750, May, 2008.
Rights and permissions
About this article
Cite this article
Potikha, L.M., Gutsul, R.M., Kovtunenko, V.A. et al. Condensed isoquinolines 30. Acylation and alkylation of 5,13-dihydro-11H-isoquino-[3,2-b]quinazolin-11-one. Chem Heterocycl Comp 44, 585–593 (2008). https://doi.org/10.1007/s10593-008-0078-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-008-0078-5