Abstract
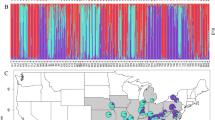
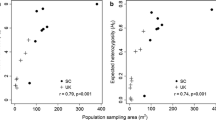
The genetic diversity of peripheral populations is potentially important to the future adaptive capacity of species, although may be difficult to predict. A large number of species-at-risk in Canada are at the northern edge of their distribution, and many of these live in fragmented habitat. We used nuclear and chloroplast markers to assess patterns of genetic diversity and differentiation within and among populations of Canadian Magnolia acuminata (Cucumber tree), an endangered species in Canada that extends as far north as the fragmented Carolinian forest in southern Ontario. We also compared the genetic composition of Canadian M. acuminata to populations sampled throughout its central distribution in the USA. We found a high proportion of shared microsatellite alleles, plus a single cpDNA haplotype, distributed throughout the entire M. acuminata range. We also found that despite occupying fragmented habitat at their range periphery, Canadian populations showed little reduction in genetic diversity relative to the USA populations, and we attribute this to effective historical dispersal in a long-lived, polyploid species. However, a combination of private alleles, genetic substructuring, and lower levels of genetic diversity in seedlings compared to mature trees, suggests that current levels of gene flow are relatively low among Canadian populations. Therefore, despite high levels of genetic diversity in Canadian M. acuminata, managers should be aware that without intervention, populations will likely become increasingly isolated and experience a reduction in genetic diversity which in turn may threaten their long-term survival in Canada.



Similar content being viewed by others
References
Ambrose JD (1990) Reproductive biology of rare Carolinian plants with regard to conservation management. In: Allen G (ed) Conserving carolinian Canada. University of Waterloo Press, Waterloo, pp 23–53
Ambrose JD, Aboud SW (1984) COSEWIC status report on the Cucumber Tree Magnolia acuminata in Canada. Committee on the Status of Endangered Wildlife in Canada
Austin JD, Lougheed SC, Neidrauer L, Chek AA, Boag PT (2002) Cryptic lineages in a small frog: the post-glacial history of the spring peeper, Pseudacris crucifer (Anura: hylidae). Mol Phyl Evol 25:316–329
Azuma H, García-Franco JG, Rico-Gray V, Thien LB (2001) Molecular phylogeny of the Magnoliaceae: the biogeography of tropical and temperate disjunctions. Am J Bot 88:2275–2285
Berens DG, Braun C, Gonzalez-Martinez SC, Griebeler EM, Nathan R, Bohning-Gaese K (2014) Fine-scale spatial genetic dynamics over the life cycle of the tropical tree Prunus africana. Heredity 113:401–407
Bernhardt P, Thien B (1987) Self-isolation and insect pollination in the primitive angiosperms: new evaluations of older hypotheses. Plant Syst Evol 156:159–176
Beresford-Kroeger D (2003) Arboretum America: a philosophy of the forest. University of Michigan Press, Ann Arbor
Birky CW (1988) Evolution and population genetics of organelle genes: mechanisms and models. In: Gottlieb LD, Jain SK (eds) Plant evolutionary biology. Chapman & Hall, London, pp 23–53
Bohonak AJ (2002) IBD (Isolation By Distance): a program for analyses of isolation by distance. J Hered 93:153–154
Chung MY, Epperson BK, Chung MG (2003) Genetic structure of age classes in Camellia japonica (Theaceae). Evolution 57:62–73
Chung MY, Nason JD, Lopez-Pujol J, Yamashiro T, Yang B-Y, Luo Y-B, Chung MG (2014) Genetic consequences of fragmentation on populations of the terrestrial orchid Cymbidium goeringii. Biol Cons 179:222–231
Church SA, Kraus JM, Mitchell JC, Church DR, Taylor DR (2003) Evidence for multiple Pleistocene refugia in the postglacial expansion of the eastern tiger salamander, Ambystoma tigrinum tigrinum. Evolution 57:372–383
Cicuzza D, Newton A, Oldfield S (2007) The red list of magnoliaceae. Fauna & Flora International, Cambridge
Ciotir C, Yesson C, Freeland JR (2013) The evolutionary history and conservation value of disjunct Bartonia paniculata subsp. paniculata (Branched Bartonia) populations in Canada. Botany 9:605–613
Cires E, De Smet Y, Cuesta C, Goetghebeur P, Sharrock S, Gibbs D, Oldfield S, Kramer A, Samain M-S (2013) Gap analyses to support ex situ conservation of genetic diversity in Magnolia, a flagship group. Biodiv Cons 22:567–590
COSEWIC (2010) COSEWIC assessment and status report on the Cucumber Tree Magnolia acuminata in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. www.sararegistry.gc.ca/status/status_e.cfm
Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A (2003) Fine-scale phylogeographical analysis of Mediterranean Anacamptis palustris (Orchidaceae) populations based on chloroplast minisatellite and microsatellite variation. Mol Ecol 12:2783–2792
Craft KJ, Ashley MV (2007) Landscape genetic structure of bur oak (Quercus macrocarpa) savannas in Illinois. For Ecol Manag 239:13–20
Dick CW, Jones FA, Hardy OH, Petit R (2008) Spatial scales of seed and pollen-mediated gene flow in tropical forest trees. Trop Plant Biol 1:20–33
Diniz-Filho JAF, Nabout JC, Bini LM, Soares TN, De Campus Telles MP, De Marco P Jr, Collevatti RG (2009) Niche modelling and landscape genetics of Caryocar brasiliense (“Pequi” tree: Caryocaraceae) in Brazilian Cerrado: an integrative approach for evaluating central-peripheral population patterns. Tree Genet Genom 5:617–627
Dong W, Liu J, Yu J, Wang L, Zhou S (2012) Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS One 7:e35071
Dufresne F, Stift M, Vergilino R, Mable BK (2011) Recent progress and challenges in population genetics of polyploid organisms: an overview of current state-of-the-art molecular and statistical tools. Mol Ecol 23:40–69
Durand E, Jay F, Gaggiotti OE (2009a) Spatial inference of admixture proportions and secondary contact zones. Mol Biol Evol 26:1963–1973
Durand E, Chen C, Francois O (2009b) Comment on ‘On the inference of spatial structure from population genetics data’. Bioinformatics 25:1802–1804
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol Ecol 17:1170–1188
Ehlers J, Gibbard P (2008) Extent and chronology of Quaternary glaciation. Episodes 31:211–218
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578
Flannery T (2003) The eternal frontier: an ecological history of North America and its peoples. Grove Press, New York
Francois O, Durand E (2010) Spatially explicit Bayesian clustering models in population genetics. Mol Ecol Res 10:773–784
Freeland JR, Vachon N (2012) Repetitive sequences in phylogeographic inference: a reply to Saltonstall and Lambertini (2012). Mol Ecol Res 12:586–589
Freeland JR, Rimmer VK, Okamura B (2004) Evidence for a residual post-glacial founder effect in a highly dispersive freshwater invertebrate. Limnol Oceanog 49:879–883
Freeland JR, Gillespie J, Ciotir C, Dorken ME (2010) Conservation genetics of Hill’s thistle (Cirsium hillii). Botany 88:1073–1080
Frodin D, Govaerts R (1996) World checklist and bibliography of Magnoliaceae. Royal Botanic Gardens, Richmond
Ghazoul J (2005) Pollen and seed dispersal among dispersed plants. Biol Rev 80:413–443
Hamilton JA, Eckert C (2007) Population genetic consequences of geographic disjunction: a prairie plant isolated on Great Lakes alvars. Mol Ecol 16:1649–1660
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New For 6:95–124
Hardy OJ, Vekemans X (2002) Spagedi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Phil Trans Roy Soc B 359:183–195
Holsinger KE, Lewis PO (2003) Hickory: a package for analysis of population genetic data version 1.1. University of Connecticut, Storrs. http://darwin.eeb.uconn.edu/hickory/hickory.html
Isagi Y, Kanazahi T, Suzuki W, Tanaka H, Abe T (1999) Polymorphic microsatellite DNA markers for Magnolia obovata Thunb. and their utility in related species. Mol Ecol 8:698–700
Jost L (2008) GST and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Keener C, Kuhns E (1997) The impact on Iroquoian populations on the northern distribution of pawpaws in the northeast. N Am Archeol 16:327–342
Kirk H, Paul J, Straka J, Freeland JR (2011) Long-distance dispersal and high genetic diversity are implicated in the invasive spread of the common reed, Phragmites australis (Poaceae) in Northeastern North America. Am J Bot 98:1180–1190
Kolpakov R, Bana G, Kucherov G (2003) mreps: efficient and flexible detection of tandem repeats in DNA. NAR 31:3672–3678
Kuang DY, Wu H, Wang YL, Gao LM, Zhang SZ, Lu L (2011) Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): implication for DNA barcoding and population genetics. Genome 54:663–673
Kuo C, Janzen F (2004) Genetic effects of a persistent bottleneck on a natural population of ornate box turtles (Terrapene ornata). Cons Gen 5:425–437
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Lindenmayer DB, Fischer J (2006) Habitat fragmentation and landscape change. Island Press, Washington, DC
Lippé C, Dumont P, Bernatchez L (2006) High genetic diversity and no inbreeding in the endangered copper redhorse, Moxostoma hubbsi (Catostomidae, Pisces): the positive sides of a long generation time. Mol Ecol 15:1769–1780
Loehle C (2007) Predicting Pleistocene climate from vegetation in North America. Clim Past 3:109–118
Lowe AJ, Boshier D, Ward M, Bacles CFE, Navarro C (2005) Genetic resource impacts of habitat loss and degradation; reconciling empirical evidence and predicted theory for neotropical trees. Heredity 95:255–273
Mahy G, Bruederle LP, Connors B, Van Hofwegen M, Vorsa N (2000) Allozyme evidence for genetic autopolyploidy and high genetic diversity in tetraploid cranberry, Vaccinium oxycoccos (Ericaceae). Am J Bot 87:1882–1889
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
McLachlan JS, Clark JS, Manos PS (2005) Molecular indicators of tree migration capacity under rapid climate change. Ecology 86:2088–2098
Meirmans PG, van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Meirmans PG, Hedrick PW (2011) Assessing population structure: F-ST and related measures. Mol Ecol Res 11:5–18
Moody ME, Mueller LD, Soltis DE (1993) Genetic variation and random drift in autotetraploid populations. Genetics 134:649–657
NatureServe (2014) NatureServe Explorer: an online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington. http://explorer.natureserve.org. Accessed 27 May 2014
Noreen AME, Webb EL (2013) High genetic diversity in a potentially vulnerable tropical tree species despite extreme habitat loss. PLoS One 8:e82632
Noss RF, Csuti B (1994) Habitat fragmentation. In: Meffe GK, Carroll CR (eds) Principles of conservation biology. Sinauer, Sunderland, pp 237–264
Obbard DJ, Harris SA, Pannell JR (2006) Simple allelic-phenotype diversity and differentiation statistics for allopolyploids. Heredity 97:296–303
Ortega J, Bonal R, Muñoz A (2010) Genetic consequences of habitat fragmentation in long-lived tree species: the case of the Mediterranean Holm Oak (Quercus ilex, L.). J Hered 101:717–726
Palop-Esteban M, Segarra-Moragues JG, Gonzalez-Candelas F (2011) Polyploid origin, genetic diversity and population structure in the tetraploid sea lavender Limonium narbonense Miller (Plumbaginaceae) from eastern Spain. Genetica 139:1309–1322
Pandey M, Rajora OP (2012) Genetic diversity and differentiation of core versus peripheral populations of eastern white cedar, Thuja occidentalis (Cupressaceae). Am J Bot 99:690–699
Paul J, Budd C, Freeland JR (2013) Conservation genetics of an endangered orchid in eastern Canada. Cons Gen 14:195–204
Petit RJ et al (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Piotti A (2009) The genetic consequences of habitat fragmentation: the case of forests. iForest 2:75–76
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Quesada M, Herrerias-Diego Y, Lobo JA, Sánchez-Montoya G, Rosas F, Aguilar R (2013) Long-term effects of habitat fragmentation on mating patterns and gene flow of a tropical dry forest tree, Ceiba aesculifolia (Malvaceae: Bombacoideae). Am J Bot 100:1095–1101
Ronfort J, Jenczewski E, Bataillon T, Rousset F (1998) Analysis of population structure in autotetraploid species. Genetics 150:921–930
Rosas F, Quesada M, Lobo JA, Sork VL (2011) Effects of habitat fragmentation on pollen flow and genetic diversity of the endangered tropical tree Swietenia humilis (Meliaceae). Biol Cons 144:3082–3088
Row JR, Brooks RJ, MacKinnon CA, Lawson A, Crother BI, White M, Lougheed SC (2011) Approximate Bayesian computation reveals the factors that influence genetic diversity and population structure of foxsnakes. J Evol Biol 24:2364–2377
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sagarin RD, Gaines SD (2002) The ‘abundant centre’distribution: to what extent is it a biogeographical rule? Ecol Lett 5:137–147
Sagarin RD, Gaines SD, Gaylord B (2002) Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol Evol 21(9):524–530
Sebbenn AM, Carvalho ACM, Freitas MLM, Moraes SMB, Gaino APSC, daSilva JM, Jolivet C, Moraes MLT (2011) Low levels of realized seed and pollen gene flow and strong spatial genetic structure in a small, isolated and fragmented population of the tropical tree Copaifera langsdorffi Desf. Heredity 106:134–145
Sewell MM, Parks CR, Chase MW (1996) Intraspecific chloroplast DNA variation and biogeography of North American Liriodendron L. (Magnoliaceae). Evolution 50:1147–1154
Shaw J, Lickey E, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL (2005) The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am J Bot 92:142–166
Shaw J, Lickey EB, Schilling EE, Small RL (2007) Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetics studies in angiosperms: the tortoise and the hare III. Am J Bot 94:275–288
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Soltis DE, Soltis PS (1993) Molecular data and the dynamic nature of polyploidy. Crit Rev Plant Sci 12:243–273
Sternberg G, Wilson J (2004) Native trees for north American landscapes. Timber Press, Portland
Strobl S, Bland D (2000) Silvicultural guide to managing southern Ontario forests. Ontario Ministry of Natural Resources, Toronto
Tollefsrud MM, Sønstebø JH, Brochmann C, Johnsen Ø, Skrøppa T, Vendramin GG (2009) Combined analysis of nuclear and mitochondrial markers provide new insight into the genetic structure of North European Picea abies. Heredity 102:549–562
Vachon N, Freeland JR (2011) Phylogeographic inferences from chloroplast DNA: quantifying the effects of mutations in repetitive and non-repetitive sequences. Mol Ecol Res 11:279–285
Verta J-P, Landry CR, MacKay JJ (2013) Are long-lived trees poised for evolutionary change? Single locus effects in the evolution of gene expression networks in spruce. Mol Ecol 22:2369–2379
Vinson CC, Kanashiro M, Harris SA, Boshier DH (2015) Impacts of selective logging on inbreeding and gene flow in two Amazonian timber species with contrasting ecological and reproductive characteristics. Mol Ecol 24:38–53
Voss N, Eckstein RL, Durka W (2012) Range expansion of a selfing polyploid plant despite widespread genetic uniformity. Ann Bot 110:585–593
Vranckx G, Jacquemyn H, Muys B, Honnay O (2012) Meta-analysis of susceptibility of woody plants to loss of genetic diversity through habitat fragmentation. Cons Biol 26:228–237
Waldron G (1993) Trees of the Carolinian forest: a guide to species, their ecology, and uses. Boston Mills Press, Erin
Weigand AM, Pfenninger M, Jochum A, Klussmann-Kolb A (2012) Alpine crossroads or origin of genetic diversity? Comparative phylogeography of two sympatric microgastropod species. PLoS One 7:e37089
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Willmer P (2011) Pollination and floral ecology. Princeton University Press, Princeton
Yakimowski SB, Eckert CG (2007) Threatened peripheral populations in context: geographical variation in population frequency and size and sexual reproduction in a clonal woody shrub, Vaccinium stamineum (Ericaceae). Cons Biol 21:811–822
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Tree 11:413–418
Acknowledgments
Many thanks to Graham Buck and Donald Kirk of the Ontario Ministry of Natural Resources for assistance with permits and location information; Danny Bernard of the Big Creek Conservation Authority for providing access to, and knowledge about, trees within the National Wildlife Area; Dave Holmes of the Long Point Conservation Authority; Deborah Dale of the North American Native Plant Society; Jennifer Paul and Adam Wilford for assistance with fieldwork; Richard Figler and members of the Magnolia Society for collecting plant tissue from USA sites. Sampling in Ontario was conducted under Permit GU-B-018-11 issued under the Endangered Species Act. Financial support was provided by an Ontario Ministry of Natural Resources Species at Risk Research Fund awarded to JF, an Ontario Ministry of Natural Resources Species at Risk Stewardship Fund awarded to JF, the Natural Sciences and Engineering Research Council (JF Discovery Grant), and Trent University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Budd, C., Zimmer, E. & Freeland, J.R. Conservation genetics of Magnolia acuminata, an endangered species in Canada: Can genetic diversity be maintained in fragmented, peripheral populations?. Conserv Genet 16, 1359–1373 (2015). https://doi.org/10.1007/s10592-015-0746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-015-0746-9