Abstract
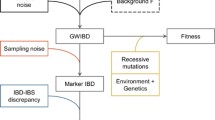
Pedigrees are frequently recommended for estimating inbreeding coefficients (F PED ), but are error-prone due to missing behavioural data in wild populations. Genetic marker-based pedigrees have been suggested as a remedy to this problem, but their accuracy depends on the number and polymorphism of loci available, and the completeness of population sampling. We used simulations to examine how accuracy of marker-based pedigrees varies with number of loci and sampling regime when allelic diversity is low (2.2–4 alleles per locus in founders), as is often the case in threatened species. We also examined the impact of pedigree errors on the validity of F PED estimated from marker-based pedigrees. Our results indicate that accurate parentage assignments are only feasible if genotypes are available for all individuals that ever existed in the population, and that accuracy does not improve past 40 loci. Errors in marker-based pedigrees resulted in underestimation of F PED by up to 27 % and overestimation of the variance in F PED by up to 182 %. At least 80 % pedigree accuracy was required to produce unbiased estimates of F PED , which were still highly imprecise. Given the degree of sampling required, it is not currently feasible to measure inbreeding in wild populations of threatened species with a pedigree based solely on microsatellite data. Resources may be better directed towards developing more robust genetic tools (whole genome sequencing and large SNP panels) to facilitate direct estimation of inbreeding coefficients without a pedigree. Where this is not possible, long-term monitoring projects will be required to accurately estimate inbreeding coefficients via a combination of behavioural and genetic data.



Similar content being viewed by others
References
Akcay E, Roughgarden J (2007) Extra-pair paternity in birds: review of the genetic benefits. Evol Ecol Res 9:855–868
Allendorf FW, Luikart G (2007) Conservation and the genetics of populations. Blackwell Publishing, Malden
Allendorf FW, Ryman N (2002) The role of genetics in population viability analysis. In: Beissinger SR, McCullough DR (eds) Population viability analysis. The Chicago University Press, Chicago, pp 50–85
Allendorf FW, Hohenlohe PA, Luikart G (2010) Genomics and the future of conservation genetics. Nat Rev Genet 11:697–709
Allendorf FW, Luikart G, Aitken SN (2013) Conservation and the genetics of populations, 2nd edn. Wiley-Blackwell Publishing, Chichester
Almudevar A (2003) A simulated annealing algorithm for maximum likelihood pedigree reconstruction. Theor Popul Biol 63:63–75
Austin JD, Johnson A, Matthews M, Tringali MD, Porak WF, Allen MS (2012) An assessment of hatchery effects on Florida bass (Micropterus salmoides floridanus) microsatellite genetic diversity and sib-ship reconstruction. Aquacult Res 43:628–638
Balloux F, Amos W, Coulson T (2004) Does heterozygosity estimate inbreeding in real populations? Mol Ecol 13:3021–3031
Bernatchez L, Duchesne P (2000) Individual-based genotype analysis in studies of parentage and population assignment: how many loci, how many alleles? Can J Fish Aquat Sci 57:1–12
Blouin MS (2003) DNA-based methods for pedigree reconstruction and kinship analysis in natural populations. Trends Ecol Evol 18:503–511
Clutton-Brock T, Sheldon BC (2010) Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol Evol 25:562–573
Colbourne RM, Robertson HA (1997) Successful translocations of little spotted kiwi (Apteryx owenii) between offshore islands of New Zealand. Notornis 44:253–258
Cope RC, Lanyon JM, Seddon JM, Pollett PK (2014) Development and testing of a genetic marker-based pedigree reconstruction system ‘PR-genie’ incorporating size-class data. Mol Ecol Res 14:857–870
Crow JF, Kimura M (1970) An introduction to population genetics theory. The Blackburn Press, Caldwell
da Silva AG, Lalonde DR, Quse V, Shoemaker A, Russello MA (2010) Genetic approaches refine ex situ lowland tapir (Tapirus terrestris) conservation. J Hered 101:581–590
Engh AL, Funk SM, Van Horn RC, Scribner KT, Bruford MW, Libants S, Szykman M, Smale L, Holekamp KE (2002) Reproductive skew among males in a female-dominated mammalian society. Behav Ecol 13:193–200
Estoup A, Gharbi K, SanCristobal M, Chevalet C, Haffray P, Guyomard R (1998) Parentage assignment using microsatellites in turbot (Scophthalmus maximus) and rainbow trout (Oncorhynchus mykiss) hatchery populations. Can J Fish Aquat Sci 55:715–725
Forstmeier W, Schielzeth H, Mueller JC, Ellegren H, Kempenaers B (2012) Heterozygosity-fitness correlations in zebra finches: microsatellite markers can be better than their reputation. Mol Ecol 21:3237–3249
Frankham R (1995) Inbreeding and extinction: a threshold effect. Conserv Biol 9:792–799
Gelatt TS, Davis CS, Stirling I, Siniff DB, Strobeck C, Delisle I (2010) History and fate of a small isolated population of Weddell seals at White Island, Antarctica. Conserv Genet 11:721–735
Gerber S, Chabrier P, Kremer A (2003) FAMOZ: a software for parentage analysis using dominant, codominant and uniparentally inherited markers. Mol Ecol Notes 3:479–481
Grueber CE, Waters JM, Jamieson IG (2011) The imprecision of heterozygosity-fitness correlations hinders the detection of inbreeding and inbreeding depression in a threatened species. Mol Ecol 20:67–79
Harrison HB, Saenz-Agudelo P, Planes S, Jones GP, Berumen ML (2013) Relative accuracy of three common methods of parentage analysis in natural populations. Mol Ecol 22:1158–1170
Heather B, Robertson H (2005) The field guide to the Birds of New Zealand. Penguin, Auckland
Hill WG, Weir BS (2011) Variation in actual relationship as a consequence of Mendelian sampling and linkage. Genet Res 93:47–64
Hoffman JI, Amos W (2005) Microsatellite genotyping errors: detection approaches, common sources and consequences for paternal exclusion. Mol Ecol 14:599–612
Jamieson IG, Wallis GP, Briskie JV (2006) Inbreeding and endangered species management: is New Zealand out of step with the rest of the world? Conserv Biol 20:38–47
Johnson JA, Tingay RE, Culver M, Hailer F, Clarke ML, Mindell DP (2009) Long-term survival despite low genetic diversity in the critically endangered Madagascar fish-eagle. Mol Ecol 18:54–63
Jones OR, Wang J (2009) Molecular marker-based pedigrees for animal conservation biologists. Anim Conserv 13:26–34
Jones OR, Wang JL (2010) COLONY: a program for parentage and sibship inference from multilocus genotype data. Mol Ecol Res 10:551–555
Jones KL, Glenn TC, Lacy RC, Pierce JR, Unruh N, Mirande CM, Chavez-Ramirez F (2002) Refining the Whooping Crane studbook by incorporating microsatellite DNA and leg-banding analyses. Conserv Biol 16:789–799
Kardos M, Allendorf FW, Luikart G (2014) Evaluating the role of inbreeding depression in heterozygosity-fitness correlations: how useful are tests for identity disequilibrium? Mol Ecol Res 14:519–530
Keller MC, Visscher PM, Goddard ME (2011) Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics 189:237–249
Koch M, Hadfield JD, Sefc KM, Sturmbauer C (2008) Pedigree reconstruction in wild cichlid fish populations. Mol Ecol 17:4500–4511
Lepais O, Darvill B, O’Connor S, Osborne JL, Sanderson RA, Cussans J, Goffe L, Goulson D (2010) Estimation of bumblebee queen dispersal distances using sibship reconstruction method. Mol Ecol 19:819–831
Malécot G (1948) Les Mathématiques de l’hérédité. Masson et Cie, Paris
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Morrissey MB, Wilson AJ (2010) Pedantics: an r package for pedigree-based genetic simulation and pedigree manipulation, characterization and viewing. Mol Ecol Res 10:711–719
Morrissey MB, Wilson AJ, Pemberton JM, Ferguson MM (2007) A framework for power and sensitivity analyses for quantitative genetic studies of natural populations, and case studies in Soay sheep (Ovis aries). J Evol Biol 20:2309–2321
Mourier J, Buray N, Schultz JK, Clua E, Planes S (2013) Genetic network and breeding patterns of a sicklefin lemon shark (Negaprion acutidens) population in the Society Islands, French Polynesia. PLoS One 8:e73899
Nielsen R, Mattila DK, Clapham PJ, Palsboll PJ (2001) Statistical approaches to paternity analysis in natural populations and applications to the North Atlantic humpback whale. Genetics 157:1673–1682
Nielsen JF, English S, Goodall-Copestake WP, Wang JL, Walling CA, Bateman AW, Flower TP, Sutcliffe RL, Samson J, Thavarajah NK, Kruuk LEB, Clutton-Brock TH, Pemberton JM (2012) Inbreeding and inbreeding depression of early life traits in a cooperative mammal. Mol Ecol 21:2788–2804
Pemberton JM (2004) Measuring inbreeding depression in the wild: the old ways are the best. Trends Ecol Evol 19:613–625
Pemberton JM (2008) Wild pedigrees: the way forward. Proc R Soc B 275:613–621
Purfield DC, Berry DP, McParland S, Bradley DG (2012) Runs of homozygosity and population history in cattle. BMC Genet 13:70
Ramstad KM, Pfunder M, Robertson HA, Colbourne RM, Allendorf FW, Daugherty CH (2010) Fourteen microsatellite loci cross-amplify in all five kiwi species (Apteryx spp.) and reveal extremely low genetic variation in little spotted kiwi (A. owenii). Conserv Genet Res 2:333–336
Ramstad KM, Colbourne RM, Robertson HA, Allendorf FW, Daugherty CH (2013) Genetic consequences of a century of protection: serial founder events and survival of the little spotted kiwi (Apteryx owenii). Proc R Soc B 280:20130576
Read KD, Lemay MA, Acheson S, Boulding EG (2012) Using molecular pedigree reconstruction to evaluate the long-term survival of outplanted hatchery-reared larval and juvenile northern abalone (Haliotis kamtschatkana). Conserv Genet 13:801–810
Reid JM, Keller LF, Marr AB, Nietlisbach P, Sardell RJ, Arcese P (2013) Pedigree error due to extra pair reproduction substantially biases estimates of inbreeding depression. Evolution 68(3):802–815
Riester M, Stadler PF, Klemm K (2009) FRANz: reconstruction of wild multi-generation pedigrees. Bioinformatics 25:2134–2139
Ringler E (2012) The use of cross-species testing of microsatellite markers and sibship analysis in ex situ population management. Conserv Genet Res 4:815–819
Robertson HA, Colbourne RM (2004) Survival of little spotted kiwi (Apteryx owenii) on Kapiti Island. Notornis 51:161–163
Robertson HA, Colbourne RM, Graham PJ, Miller PJ, Pierce RJ (2011) Experimental management of Brown Kiwi Apteryx mantelli in central Northland, New Zealand. Bird Conserv Int 21:207–220
Robinson SP, Simmons LW, Kennington WJ (2013) Estimating relatedness and inbreeding using molecular markers and pedigrees: the effect of demographic history. Mol Ecol 22:5779–5792
Russello MA, Amato G (2004) Ex situ population management in the absence of pedigree information. Mol Ecol 13:2829–2840
Santure AW, Stapley J, Ball AD, Birkhead TR, Burke T, Slate J (2010) On the use of large marker panels to estimate inbreeding and relatedness: empirical and simulation studies of a pedigreed zebra finch population typed at 771 SNPs. Mol Ecol 19:1439–1451
Slate J, David P, Dodds KG, Veenvliet BA, Glass BC, Broad TE, McEwan JC (2004) Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93:255–265
Taylor HR (2014) Detecting inbreeding depression in a severely bottlenecked, recovering species: the little spotted kiwi (Apteryx owenii): a thesis submitted to the Victoria University of Wellington in fulfilment of the requirements for the degree of Doctor of Philosophy in Ecology and Biodiversity. Victoria University of Welligton
Taylor SS, Sardell RJ, Reid JM, Bucher T, Taylor NG, Arcese P, Keller LF (2010) Inbreeding coefficient and heterozygosity-fitness correlations in unhatched and hatched song sparrow nestmates. Mol Ecol 19:4454–4461
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria
Therneau T, Atkinson E, Sinnwell J, Matsumoto M, Schaid D, McDonnell S (2011) kinship2: Pedigree functions
Thomas SC (2005) The estimation of genetic relationships using molecular markers and their efficiency in estimating heritability in natural populations. Phil Trans R Soc B 360:1457–1467
Ursprung E, Ringler M, Jehle R, Hodl W (2011) Strong male/male competition allows for non choosy females: high levels of polygynandry in a territorial frog with paternal care. Mol Ecol 20:1759–1771
Wang J (2006) Informativeness of genetic markers for pairwise relationship and relatedness inference. Theor Popul Biol 70:300–321
Wang J (2007) Triadic IBD coefficients and applications to estimating pairwise relatedness. Genet Res 89:135–153
Wang JL (2011) COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol Ecol Res 11:141–145
Wang J (2014) Marker-based estimates of relatedness and inbreeding coefficients: an assessment of current methods. J Evol Biol 27:518
Wang J, Santure AW (2009) Parentage and sibship inference from multilocus genotype data under polygamy. Genetics 181:1579–1594
Wright S (1922) Coefficients of inbreeding and relationship. Am Nat 56:330–338
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
The authors thank Jinliang Wang for his advice regarding the program COLONY, Kevin Buckley for his assistance with the Victoria University Condor computing platform and Nicola Nelson plus two anonymous reviewers for helpful comments on earlier versions of this manuscript. This study was funded by the Allan Wilson Centre, Victoria University of Wellington, the Centre for Biodiversity and Restoration Ecology, the New Zealand Ministry for Business, Innovation and Employment, and Kaitiaki o Kapiti Trust.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Taylor, H.R., Kardos, M.D., Ramstad, K.M. et al. Valid estimates of individual inbreeding coefficients from marker-based pedigrees are not feasible in wild populations with low allelic diversity. Conserv Genet 16, 901–913 (2015). https://doi.org/10.1007/s10592-015-0709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-015-0709-1