Abstract
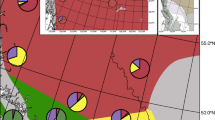
Hybridization is an evolutionary mechanism capable of enhancing adaptive potential, especially among species in fragmented or disturbed ecosystems like coastal marshes. In this study, we evaluated whether hybridization might influence adaptive responses in coastal marshes that are susceptible to the effects of global environmental change. To do so, we examined the extent and nature of hybridization between Schoenoplectus americanus and S. pungens, two ecologically dominant sedges in low-lying marshes across Chesapeake Bay (USA). Observed patterns of variation at genetically based morphological traits, cpDNA and nuclear microsatellite markers confirm that introgressive hybridization occurs between the two species. Comparisons of microsatellite and cpDNA profiles found that hybridization is reciprocal, although a disproportionate number of hybrids exhibit genomic asymmetries favoring S. americanus. AIC model selection consistently identified latitude as the strongest explanatory variable for the distribution of parental species, although discriminant analysis indicated that distributions also correspond to variation in environmental conditions. Discriminant analysis further indicated that ecological correlates of hybrid and S. americanus genotypes are similar, but not uniformly so. These findings indicate that the boundary between S. americanus and S. pungens is porous, and that hybridization could influence responses of one or both species to changing environmental regimes.






Similar content being viewed by others
References
Abbott R (1992) Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol Evol 7:401–405
Anderson EC, Thompson EA (2002) A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160:1217–1229
Anttila CK, Daehler CC, Rank NE, Strong DR (1998) Greater male fitness of a rare invader (Spartina alterniflora, Poaceae) threatens a common native (Spartina foliosa) with hybridisation. Am J Bot 85:1597–1601
Arnold ML (1997) Natural hybridization and evolution. Oxford University Press, New York
Arnold ML, Hodges SA (1995) Are natural hybrids fit or unfit relative to their parents? Trends Ecol Evol 10:67–71
Arp WJ, Drake BG, Pockman WT, Curtis PS, Whigham DF (1993) Interactions between C3 and C4 salt-marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetation 104:133–143
Ayres DR, Strong DR (2001) Origin and genetic diversity of Spartina anglica C. E. Hubbard (Poaceae). Am J Bot 88:1863–1867
Ayres DR, Garcia-Rossi D, Davis HG, Strong DR (1999) Extent and degree of hybridization between exotic (Spartina alterniflora) and native (S. foliosa) cordgrass (Poaceae) in California, USA determined by random amplified polymorphic DNA (RAPDs). Mol Ecol 8:1179–1186
Ayres DR, Zaremba K, Sloop CM, Strong DR (2008a) Sexual reproduction of cordgrass hybrids (Spartina foliosa × alterniflora) invading tidal marshes in San Francisco Bay. Divers Distrib 14:187–195
Ayres DR, Grotkopp E, Zaremba K, Sloop CM, Blum MJ, Bailey J, Anttila C, Strong DR (2008b) Hybridization between invasive Spartina densiflora (Poaceae) and native S. foliosa in San Francisco Bay, California, USA. Am J Bot 95:713–719
Balanyá J, Oller JM, Huey RB, Gilchrist GW, Serra L (2006) Global genetic change tracks global climate warming in Drosophila subobscura. Science 313:1773–1775
Barton NH, Hewitt GM (1985) Analysis of hybrid zones. Annu Rev Ecol Syst 16:113–148
Barton NH, Hewitt GM (1989) Adaptation, speciation and hybrid zones. Nature 341:497–503
Bertness MD, Ewanchuk PJ, Silliman BR (2001) Anthropogenic modification of New England salt marsh landscapes. Proc Nat Acad Sci USA 99:1395–1398
Blum MJ (2008) Ecological and genetic associations across a Heliconius hybrid zone. J Evol Biol 21:330–341
Blum MJ, Mclachlan JS, Saunders CJ, Herrick JD (2005) Characterization of microsatellite loci in Schoenoplectus americanus (Cyperaceae). Mol Ecol Notes 5:661–663
Blum MJ, Bando J, Katz M, Strong DR (2007) Geographic structure, genetic diversity and source tracking of Spartina alterniflora. J Biogeogr 34:2055–2069
Boecklen WJ, Howard DJ (1997) Genetic analysis of hybrid zones: numbers of markers and power of resolution. Ecology 78:2611–2616
Bradshaw WE, Holzapfel CM (2006) Evolutionary responses to rapid climate change. Science 312:1477–1478
Burke JM, Carney SE, Arnold ML (1998) Hybrid fitness in the Louisiana irises: analysis of parental and F1 performance. Evolution 52:37–43
Burnham KP, Anderson DR (2002) Model selection and inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, Inc, New York
Davis MB, Shaw RG, Etterson JR (2005) Evolutionary responses to changing climate. Ecology 86:1704–1714
Department of Natural Resources State of Maryland (2008) Eyes on the Bay: fixed station monthly monitoring. http://mddnr.chesapeakebay.net/eyesonthebay/index.cfm. Accessed 25 May 2008
Dieringer D, Schlotterer C (2003) Microsatellite analyser (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Donnelly JP, Bertness MD (2001) Rapid shoreward encroachment of salt marsh cordgrass in response to accelerated sea-level rise. Proc Nat Acad Sci USA 98:14218–14223
Dowling TE, Secor CL (1997) The role of hybridization and introgression in the diversification of animals. Annu Rev Ecol Syst 28:593–619
Endler JA (1977) Geographical variation, speciation, and clines. Princeton University Press, Princeton
Engelhardt KAM, Ritchie ME (2001) Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411:687–689
Epifanio JM, Philipp DP (1997) Sources for misclassifying genealogical origins in mixed hybrid populations. J Hered 88:62–65
Etterson JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 292:151–154
Field DL, Ayre DJ, Whelan RJ, Young AG (2008) Relative frequency of sympatric species influences rates of interspecific hybridization, seed production, and seedling performance in the uncommon Eucalyptus aggregata. J Ecol 96:1198–1210
Flora of North America Editorial Committee (Eds). (1993). Flora of North America North of Mexico, vol 15. New York, Oxford
Franks SJ, Sims S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Nat Acad Sci USA 104:1278–1282
Fritsche F, Kaltze O (2000) Is the Prunella (Lamiaceae) hybrid zone structured by an environmental gradient? Evidence from a reciprocal transplant experiment. Am J Bot 87:995–1003
Gee J (2004) Gene flow across a climatic barrier between hybridizing avian species, California and Gambel’s quail (Callipepla californica and C. gambelii). Evolution 58:1108–1121
Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J (2008) Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol 17:167–178
Grant PR, Grant R (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711
Grant PR, Grant R, Markert JA, Keller LF, Petren K (2004) Convergent evolution of Darwin’s finches caused by introgressive hybridization and selection. Evolution 58:1588–1599
Hall R, Hastings AJ, Ayres DR (2006) Explaining the explosion: modelling a hybrid invasion. Proc R Soc Lond B Biol Sci 273:1385–1389
Hänfling B, Bolton P, Harley M, Carvalho GR (2005) A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshw Biol 50:403–417
Hurlbut D, Best A, Monfils AK (2007) Molecular and morphological variation in the Schoenoplectus pungens species complex (Cyperaceae). Botany and plant pathology joint congress, 2007 July 7–11, Chicago, Illinois
Ikegami M (2004) Functional specialization of Ramets in a clonal plant network. Dissertation, Utrecht University
Ikegami M, Whigham DF, Werger MJA (2009) Ramet phenology and clonal architectures of the clonal sedge Schoenoplectus americanus (Pers.) Volk. ex Schinz & R. Keller****. Plant Ecol 200:287–301
Jiggins CD, Mallet J (2000) Bimodal hybrid zones and speciation. Trends Ecol Evol 15:250–255
Johnston JA, Wesselingh RA, Bouck AC, Donovan LA, Arnold ML (2001) Intimately linked or hardly speaking? The relationship between genotype and environmental gradients in a Louisiana Iris hybrid population. Mol Ecol 10:673–681
Jump AS, Peñuelas J (2005) Running to stand still: adaptation and response of plants to rapid climate change. Ecol Lett 8:1010–1020
Kearney MS, Grace RE, Stevenson JC (1988) Marsh loss in Nanticoke estuary, Chesapeake Bay. Geogr Rev 78:205–220
Koyama T (1962) The genus Scirpus Linn.: some North American aphylloid species. Can J Bot 40:913–937
Lepais O, Petit RJ, Guichoux E, Lavabre JE, Alberto F, Kremer A, Gerber S (2009) Species relative abundance and direction of introgression in oaks. Mol Ecol 18:2228–2242
Lewontin RC, Birch LC (1966) Hybridization as a source of variation for adaptation to new environments. Evolution 20:315–336
Lynch M, Lande R (1993) Evolution and extinction in response to environmental change. In: Kareiva P, Kingsolver J, Huey R (eds) Biotic interactions and global change. Sinauer, Sunderland, Massachusetts, pp 234–250
Mallet J (2005) Hybridization as an invasion of the genome. Trends Ecol Evol 20:229–237
Moore WS (1977) An evaluation of narrow hybrid zones in vertebrates. Q Rev Biol 52:263–277
Morris JT, Sundareshwar PV, Nietch CT, Kjerfve B, Cahoon DR (2002) Responses of coastal wetlands to rising sea level. Ecology 83:2869–2877
Nolte AW, Freyhof J, Stemshorn KC, Tautz D (2005) An invasive lineage of sculpins, Cottus sp. (Pisces, Teleostei) in the Rhine with new habitat adaptations has originated from hybridization between old phylogeographic groups. Proc R Soc B 272:2379–2387
Palmisano AW, Newsom JD (1968) Ecological factors affecting occurrence of Scirpus olneyi and Scirpus robustus in the Louisiana coastal marshes. Twenty-first annual conference of southeastern association of game and fish commissions, vol 21. pp 161–172
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rasse DP, Peresta G, Drake BG (2005) Seventeen years of elevated CO2 exposure in a Chesapeake Bay wetland: sustained by contrasting responses of plant growth and CO2 uptake. Global Change Biol 11:369–377
Reusch TBH, Wood TE (2007) Molecular ecology of global change. Mol Ecol 16:3973–3992
Rhymer J, Simberloff D (1996) Extinction by hybridization and introgression. Annu Rev Ecol Syst 27:83–109
Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28:359–389
Rieseberg LH, Ellstrand N (1993) What can molecular and morphological markers tell us about plant hybridization? Crit Rev Plant Sci 12:213–241
Rieseberg LH, Wendel JF (1993) Introgression and its consequences in plants. In: Harrison RG (ed) Hybrid zones and the evolutionary process. Oxford University Press, New York, pp 70–101
Rieseberg LH, Archer MA, Wayne RK (1999) Transgressive segregation, adaptation and speciation. Heredity 83:363–372
Rieseberg LH, Kim S, Randell RA, Whitney KD, Gross BL, Lexer C, Clay K (2007) Hybridization and the colonization of novel habitats by annual sunflowers. Genetica 129:149–165
Saunders CJ (2003) Soil accumulation in a Chesapeake Bay salt marsh: modeling 500 years of global change, vegetation change, and rising atmospheric CO2. Dissertation, Duke University
Seehausen O (2004) Hybridization and adaptive radiation. Trends Ecol Evol 19:198–207
Seehausen O, van Alphen JJM, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Smith G (1995) New combinations in North American Schoenoplectus, Bolboschoenus, Isolepis, and Trichophorum (Cyperaceae). Novon 5:97–102
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Taylor EB, Boughman JW, Groenenboom M, Sniatynski M, Schluter D, Gow JL (2006) Speciation in reverse: morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Mol Ecol 15:343–355
Umina PA, Weeks AR, Kearney MR, McKechnie SW, Hoffman AA (2005) A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 308:691–693
Walters DM, Blum MJ, Rashleigh B, Freeman BJ, Porter BA, Burkhead NM (2008) Red shiner invasion and hybridization with blacktail shiner in the upper Coosa River, USA. Biol Invasions 10:1229–1242
Wang H, McArthur ED, Sanderson SC, Graham JH, Freeman DC (1997) Narrow hybrid zone between two subspecies of big sagebrush (Artemisia tridentata: Asteraceae). IV. Reciprocal transplant experiments. Evolution 51:95–102
Warren RS, Niering WA (1993) Vegetation change on a northeast tidal marsh: interaction of sea-level rise and marsh accretion. Ecology 74:96–103
Whitham TG, Bailey JK, Schweitzer JA et al (2006) A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Genet 7:510–523
Wray RD, Leatherman SP, Nicholls RJ (1995) Historic and future land loss for upland and marsh islands in the Chesapeake Bay, Maryland, USA. J Coast Res 11:1195–1203
Acknowledgements
The authors thank Mark Fox for preparing illustrations for this manuscript. Ashley Kuenzi also provided generous laboratory assistance. We thank members of the Blum laboratory and two anonymous reviewers for helpful editorial advice. This research was funded by the U.S. Environmental Protection Agency (Contract #4D-5709-NAEX) and Tulane University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blum, M.J., Knapke, E., McLachlan, J.S. et al. Hybridization between Schoenoplectus sedges across Chesapeake Bay marshes. Conserv Genet 11, 1885–1898 (2010). https://doi.org/10.1007/s10592-010-0080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0080-1