Abstract
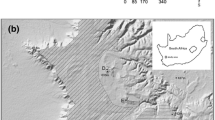
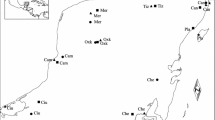
Habitat fragmentation is believed to be a key threat to biodiversity, with habitat specialists being stronger affected than generalists. However, pioneer species might be less affected by fragmentation, as their high colonization potential should increase gene flow. Here, we present an analysis of the genetic structure of populations of the solitary bee Andrena vaga, which naturally occurs in sandy habitats and is specialized on willow (Salix) pollen as larval food and sandy soils as nesting sites. While the species is widespread in the young sandy landscapes of our main study area (Emsland, northwestern Germany), it occurs less frequently in the Lower Rhine valley. Our analyses of six polymorphic microsatellites show that the populations are only slightly differentiated, suggesting a relatively strong gene flow. No genetic structure corresponding to the geographic origin was found as the variability within populations accounted for the major proportion of variation. FST values were higher and allelic richness was lower in the Lower Rhine valley, supporting the hypothesis that habitat availability affects the degree of genetic exchange between populations. Inbreeding coefficients were generally high and nearly all populations had a heterozygote deficiency, which could be explained by the breeding strategy of A. vaga, which nests in aggregations.




Similar content being viewed by others
References
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settle J, Kunin WE (2006) Parallel decline in pollinators and insect pollinated plants in Britain and the Netherlands. Science 313:351–354
Bischoff I (2003) Population dynamics of the solitary digger bee Andrena vaga Panzer (Hymenoptera, Andrenidae) studied using mark-recapture and nest counts. Pop Ecol 45:197–204
Bohonak AJ (2002) IBD (Isolation by Distance): a program for analysis of isolation by distance. J Hered 93:153–154
Bonte D, Baert L, Lens L, Maelfait J-P (2004) Effects of aerial dispersal, habitat specialisation, and landscape structure on spider distribution across fragmented grey dunes. Ecography 27:343–349
Butler SJ, Vickery JA, Norris K (2007) Farmland biodiversity and the footprint of agriculture. Science 315:381–384
Callen DF, Thompson AD, Shen Y, Phillips HA, Richards RI, Mulley JC, Sutherland GR (1993) Incidence and origin of “null” alleles in the (AC)n microsatellite Markers. Am J Hum Genet 52:922–927
Cane JH, Tepedino VJ (2001) Causes and extent of declines among native North American invertebrate pollinators: detection, evidence, and consequences. Conservation Ecology 5(1):1 [online] URL: http://www.ecologyandsociety.org/vol5/iss1/art1/
Chapman RE, Wang J, Bourke AFG (2003) Genetic analysis of spatial foraging patterns and resource sharing in bumble bee pollinators. Mol Ecol 12:2891–2808
Danforth BN, Shuqing J, Ballard LJ (2003) Gene flow and population structure in an oligolectic desert bee, Macrotera (Macroteropsis) portalis (Hymenoptera: Andrenidae) J Kans Entomol Soc 76:221–235
Darvill B, Ellis JS, Lye GC, Goulson D (2006) Population structure and inbreeding in a rare and declining bumblebee, Bombus muscorum (Hymenoptera: Apidae). Mol Ecol 15:601–611
Ellis JS, Knight ME, Darvill B, Goulson D (2006) Extremely low effective population sizes, genetic structuring and reduced genetic diversity in a threatened bumblebee species, Bombus sylvarum (Hymenoptera: Apidae). Mol Ecol 15:4375–4386
El Mousadik A, Petit RJ, (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fellendorf M, Mohra C, Paxton RJ (2004) Devasting effects of river flooding to the ground nesting bee, Andrena vaga (Hymenoptera: Andrenidae), and its associated fauna. J Insect Conserv 8:311–322
Francisco FO, Brito RM, Arias MC (2006) Allele number and heterozygosity for microsatellite loci in different stingless bee species (Hymenoptera: Apidae, Meliponini). Neotrop Entomol 35:638–643
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 71:757–764
Goudet J, Raymond M, Demeeus T, Rousset F, (1996) Testing differentiation in diploid populations. Genetics 144:1933–1940
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F statistics. J Hered 86:485–486
Goulson D (2003) Conserving wild bees for crop pollination. Food Agricul Environ 1:142–144
Hanski I (2005) Landscape fragmentation, biodiversity loss and the societal response. EMBO Reports 6:388–392
Haper GL, Maclean N, Goulson D (2003) Microsatellite markers to assess the influence of population size, isolation and demographic change on the genetic structure of the UK butterfly Polyommatus bellargus. Mol Ecol 12:3349–3357
Hedrick PW, Kalinowski ST (2000) Inbreeding depression in conservation biology. Annu Rev Ecol Evol Syst 31:139–162
Henle K, Lindenmayer DB, Margules CR, Saunders DA, Wissel C (2004) Species survival in fragmented landscapes: Where are we now? Biodivers Conserv 13:1–8
Kelley ST, Farrell BD, Mitton JB (2000) Effects of specialization on genetic differentiation in sister species of bark beetles. J Hered 84:218–227
Kitahara M, Fujii K (1994) Biodiversity an community structure of temperate butterfly species within a gradient of human disturbance: an analysis based on the concept of generalist vs. specialist strategies. Res Pop Ecol 36:187–199
Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, Sanderson RA, Goulson D, (2005) An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Mol Ecol 14:1811–1820
Kratochwil A (2003) Bees (Hymenoptera: Apoidea) as keystone species: specifics of resource and requisite utilisation in different habitat types. Ber Reinh Tüxen-Ges 15:59–77
Kuhlmann M (1999) Rote Liste der gefährdeten Stechimmen (Wildbienen und Wespen, Hymenoptera Aculeata) Westfalens. 1. Fassung. In: Rote Liste der gefährdeten Pflanzen und Tiere in NRW. Schr.R 17:563–574 LÖBF, Recklinghausen
Le Corre V, Kremer A (1998) Cumulative effects of founding events during colonization on genetic diversity and differentiation in an island and stepping-stone model. J Evol Biol 11:495–512
Mohra C, Fellendorf M, Segelbacher G, Paxton RJ (2000) Dinucleotide microsatellite loci for Andrena vaga and other andrenid bees from non-enriched and CT-enriched libraries. Mol Ecol 9:2189–2191
McKinney ML (1997) Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu Rev Ecol Evol Syst 28:495–516
Osborne JL, Clark SJ, Morris RJ, Williams IH, Riley JR, Smith AD, Reynolds DR, Edwards SA (1999) A landscape-scale study of bumble bee foraging range and constancy, using harmonic radar. J Appl Ecol 36:519–533
Packer L, Owen R (2001) Population genetic aspects of pollinator decline. Conserv Ecol 5(1): 4. [online] URL: http://www.ecologyandsociety.org/vol5/iss1/art4/
Packer L, Zayed A, Grixti JC, Ruz L, Owen RE, Vivallo F, Toro H (2005) Conservation genetics of potentially endangered mutualisms: reduced levels of genetic variation in specialist versus generalist bees. Conserv Biol 19:195–202
Paxton RJ, Tengö J (1996) Intranidal mating, emergence, and sex ratio in a communal bee Andrena jacobi Perkins 1921 (Hymenoptera: Andrenidae). J Insect Behav 9:421–438
Paxton RJ, Thorén PA, Tengö J, Estoup A, Pamilo P (1996) Mating structure and nestmate relatedness in a communal bee, Andrena jacobi (Hymenoptera, Andrenidae), using microsatellites. Mol Ecol 5:511–519
Paxton RJ (2005) Male mating behaviour and mating systems of bees: an overview. Apidologie 36:145–156
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peterson MA, Denno R (1998) The influence of dispersal and diet breadth on patterns of genetic isolation by distance in phytophagous insects. Am Nat 152:428–446
Petit E, Balloux F, Goudet J (2001) Sex biased dispersal in a migratory bat: a characterization using sex-specific demographic parameters. Evolution 55:635–640
Polus E, Vandewoestijne S, Choutt J, Baguette M (2006) Tracking the effects of one century of habitat loss and fragmentation on calcareous grassland butterfly communities. Biodivers Conserv 16:3423–3436
Primack R (2002) Essentials of conservation biology. 3rd edn. Sinauer Associates, Sunderland
Raymond M, Rousset F (1995) Genepop (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
R Development Core Team 2007. R: A language and environment for statistical computing. http://www.R-project.org
Repaci V, Stow AJ, Briscoe DA (2006) Fine-scale genetic structure, co-founding and multiple mating in the Australian allodapine bee (Exoneura robusta). J Zool 270:687–691
Sallé A, Arthofer W, Lieutier F, Stauffer C, Kerdelhué C (2007) Phylogeography of a host-specific insect: genetic structure of Ips typographus in Europe does not reflect past fragmentation of its host. Biol J Linn Soc 90:239–246
Schmitt T, Hewitt GM (2004) The genetic pattern of population threat and loss: a case study of butterflies. Mol Ecol 13:21–31
Stahlhut JK, Cowan DP (2004) Inbreeding in a natural population of Euodynerus foraminatus (Hymenoptera: Vespidae), a solitary wasp with single-locus complementary sex determination. Mol Ecol 13:631–638
Steffan-Dewenter I (2003) Importance of habitat area and landscape context for species for species richness of bees and wasps in fragmented orchard meadows. Conserv Biol 17:1036–1044
Stow A, Silberbauer L, Beattie AJ, Briscoe DA (2007) Fine-scale genetic structure and fire-created habitat patchiness in the Australian allodapine bee, Exoneura nigrescens (Hymenoptera: Apidae). J Hered 98:60–66
Stroh M, Kratochwil A, Remy D, Zimmermann K, Schwabe A (2005) Rehabilitation of alluvial landscapes along the River Hase (Ems river basin, Germany). Arch Hydrobiol Suppl Large rivers 15:243–260
Theunert R (2002) Rote Liste der in Niedersachsen und Bremen gefährdeten Wildbienen mit Gesamtverzeichnis. Inform Naturschutz Niedersachs 22:138–160
Tscharntke T, Brandl R 2004 Plant-Insect Interactions in Fragmented Landscapes. Annu Rev Entomol 49:405–430
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: A mini review. Ecol Res 17:229–239
Vandewoestijne S, Nève G, Baguette M (1999) Spatial and temporal population genetic structure of the butterfly Aglais urticae L. (Lepidoptera, Nymphalidae). Mol Ecol 8:1539–1543
Van Dijk GM, Martijn ECL, Schulte-Wülwer-Ludwig A (2006) Ecological rehabilitation of the River Rhine: Plans, progress and perspectives. Reg Riv Res Manag 11:377–388
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting errors in microsatellite data. Mol Ecol Notes 4:535–538
Westrich P (1989) Die Wildbienen Baden Württembergs. Eugen Ulmer, Stuttgart
Williams NM, Kremen C (2007) Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol Appl 17:910–921
Winfree R, Griswold T, Kremen C (2007) Effect of human disturbance on bee communities in a forested ecosystem. Conserv Biol 21:213–223
Wright S (1951) The genetic structure of populations. Ann Eugen 15:323–354
Zavodna M, Arens P, Van Dijk PJ, Partomihardjo T, Vosman B, Van Damme JMM (2005) Pollinating fig wasps: genetic consequences of island recolonization. J Evol Biol 18:1234–1243
Zayed A, Packer L, Grixti JC, Ruz L, Owen RE, Toro H (2005) Increased genetic differentiation in a specialist versus a generalist bee: implications for conservation. Conserv Genet 6:1017–1026
Acknowledgements
We are grateful to I. Bischoff, M. Beil and O. Diestelhorst for collecting individuals of Andrena vaga in southern Germany. We thank L. Köster and R. Koller for assistance in the laboratory and especially U. Coja for genetic analysis. We also wish to thank R. Paxton for helpful comments. We are grateful to the Division of Ecology at the University of Osnabrück for providing research facilities. Financial support was provided by the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt). We thank the regional administrations for the permission to collect specimens of Andrena vaga.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Exeler, N., Kratochwil, A. & Hochkirch, A. Strong genetic exchange among populations of a specialist bee, Andrena vaga (Hymenoptera: Andrenidae). Conserv Genet 9, 1233–1241 (2008). https://doi.org/10.1007/s10592-007-9450-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-007-9450-8