Abstract
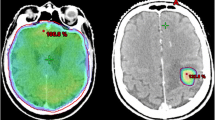
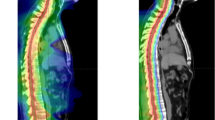
Single stereotactic radiosurgery (SRS) for posterior fossa brain metastases (BM) larger than 4cm3 is dangerous. ‘Sandwich treatment’ strategy was developed for these BMs. The strategy was one week treatment course which includes 2-stage SRS and using Bevacizumab once during SRS gap. Patients from four gamma knife center were retrospectively analyzed. The changes of tumor and peri-tumor edema volume were studied. The Dizziness Handicap Inventory (DHI) Vomiting Score (VS) and Glasgow Coma Scale (GCS) were used to evaluate patients’ clinical symptom changes. Karnofsky performance scale (KPS) and Barthel Index (BI) were used to evaluate patients’ overall fitness status and physical activity rehabilitation. Tumor local control (TLC) and patients’ overall survival (OS) rate were also calculated. Forty patients with 45 LBMs received ‘Sandwich treatment’. The mean edema volume reduced remarkably at the course of therapy and 3 months later (P < 0.01). The mean tumor volume greatly decreased 3 months later (P < 0.01). Patients’ clinical symptoms that reflected by median score of DHI, VS, GCS were improved dramatically at the course of therapy and 3 months later (P < 0.01). Similar changes happened in median score of KPS and BI that reflected patients’ overall fitness status and physical activity rehabilitation (P < 0.01). Patients’ median OS was 14.3 months, with 95.4%, 76.2%, and 26.3% survival rate at 6, 12, 24 months. The TLC rate at 6, 12, 24 months was 97.5%, 86.0% and 62.2%.The ‘Sandwich treatment’ is safe and effective for patients with LBM over 4cm3 in the posterior fossa. The strategy could quickly improve patients’ symptoms, well control tumor growth, prolong patient’s OS, and has controllable side effects.





Similar content being viewed by others
Data Availability
The data will be made available upon reasonable request.
References
Arvold ND, Lee EQ, Mehta MP et al (2016) Updates in the management of brain metastases. Neuro Oncol 18:1043–1065
Chao ST, De Salles A, Hayashi M et al (2018) Stereotactic radiosurgery in the management of limited (1–4) brain metasteses: systematic review and International Stereotactic Radiosurgery Society practice guideline. Clin Neurosurg 83:345–353
Ebner D, Rava P, Gorovets D et al (2015) Stereotactic radiosurgery for large brain metastases. J Clin Neurosci 22:1650–1654. https://doi.org/10.1016/j.jocn.2015.05.019
Serizawa T, Higuchi Y, Yamamoto M et al (2019) Comparison of treatment results between 3- and 2-stage Gamma Knife radiosurgery for large brain metastases: a retrospective multi-institutional study. J Neurosurg 131:227–237. https://doi.org/10.3171/2018.4.JNS172596
Dohm AE, Hughes R, Wheless W et al (2018) Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol 140:749–756. https://doi.org/10.1007/s11060-018-03008-8
Angelov L, Mohammadi AM, Bennett EE et al (2018) Impact of 2-staged stereotactic radiosurgery for treatment of brain metastases ≥ 2 cm. J Neurosurg 129:366–382. https://doi.org/10.3171/2017.3.JNS162532
Hasegawa T, Kato T, Yamamoto T et al (2017) Multisession gamma knife surgery for large brain metastases. J Neurooncol 131:517–524. https://doi.org/10.1007/s11060-016-2317-4
Yomo S, Hayashi M (2014) A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma Knife stereotactic radiosurgery. https://doi.org/10.1186/1748-717X-9-132. Radiat Oncol 9.
Linskey ME, Andrews DW, Asher AL et al (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68
Hawasli AH, Rubin JB, Tran DD et al (2013) Antiangiogenic agents for nonmalignant brain tumors. J Neurol Surgery Part B Skull Base 74:136–141. https://doi.org/10.1055/s-0033-1338262
Alanin MC, Klausen C, Caye-Thomasen P et al (2016) Effect of bevacizumab on intracranial meningiomas in patients with neurofibromatosis type 2 – a retrospective case series. Int J Neurosci 126:1002–1006. https://doi.org/10.3109/00207454.2015.1092443
Zamyslowska-Szmytke E, Politanski P, Jozefowicz-Korczynska M (2021) Dizziness handicap inventory in clinical evaluation of dizzy patients. Int J Environ Res Public Health 18:1–12. https://doi.org/10.3390/ijerph18052210
Schiller K, Specht HM, Haller B et al (2017) Correlation between delivered radiation doses to the brainstem or vestibular organ and nausea & vomiting toxicity in patients with head and neck cancers - an observational clinical trial. https://doi.org/10.1186/s13014-017-0846-4. Radiat Oncol 12.
Hankemeier A, Rollnik JD (2015) The early functional abilities (EFA) scale to assess neurological and neurosurgical early rehabilitation patients. BMC Neurol 15. https://doi.org/10.1186/s12883-015-0469-z
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278
Dohm A, McTyre ER, Okoukoni C et al (2018) Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Clin Neurosurg 83:114–121. https://doi.org/10.1093/neuros/nyx355
Damron EP, Dono A, Chafi H et al (2022) Metastatic neoplasm volume kinetics following 2-Stage stereotactic radiosurgery. World Neurosurg 161:e210–e219. https://doi.org/10.1016/j.wneu.2022.01.109
Hori Y, Muhsen B, Joshi K RADT-01. THE EFFICACY AND, SAFETY OF TWO-STAGED STEREOTACTIC, RADIOSURGERY FOR LARGE POSTERIOR FOSSA METASTASES: POST-TREATMENT VOLUMETRIC CHANGES IN TUMOR SIZE (2020), PERI-TUMORAL EDEMA, AND FOURTH VENTRICLE. Neuro Oncol 22:ii181–ii181. https://doi.org/10.1093/neuonc/noaa215.756
Ito D, Aoyagi K, Nagano O et al (2020) Comparison of two-stage Gamma Knife radiosurgery outcomes for large brain metastases among primary cancers. J Neurooncol 147:237–246. https://doi.org/10.1007/s11060-020-03421-y
Cho A, Medvedeva K, Kranawetter B, Untersteiner H, Hirschmann D, Lepilina O, Baulin A, Buschmann M, Ertl A, Marik W, Dorfer C, Rössler K, Gatterbauer B, Ilyalov SFJ (2022) How to dose-stage large or high-risk brain metastases: an alternative two-fraction radiosurgical treatment approach. J Neuro surg 137:1666–1675. https://doi.org/10.3171/2022.2.JNS212440
Delishaj D, Ursino S, Pasqualetti F et al (2017) Bevacizumab for the treatment of Radiation-Induced cerebral necrosis: a systematic review of the literature. J Clin Med Res 9:273–280. https://doi.org/10.14740/jocmr2936e
Erpolat OP, Demircan NV, Sarıbas GS et al (2020) A comparison of Ramipril and Bevacizumab to Mitigate Radiation-Induced Brain necrosis: an experimental study. World Neurosurg 144:e210–e220. https://doi.org/10.1016/j.wneu.2020.08.081
Lubelski D, Abdullah KG, Weil RJ, Marko NF (2013) Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol 115:317–322
Li J, He J, Cai L, Lai M, Hu Q, Ren C, Wen L, Wang J, Zhou J, Zhou Z, Li S, Ye M, Shan C, Longhua Chen CZ (2021) Bevacizumab as a treatment for radiation necrosis following stereotactic radio surgery for brain metastases clinical and radiation dosimetric impacts. Ann Palliat Med 10:2018–2026. https://doi.org/10.21037/apm-20-2417
Kraft J, van Timmeren JE, Frei S, Mayinger M, Borsky K, Kirchner C, Stark LS, Tanadini-Lang S, Wolpert F, Weller M, Woodruff HC, Guckenberger MAN (2022) Comprehensive summary and retrospective evaluation of prognostic scores for patients with newly diagnosed brain metastases treated with upfront radiosurgery in a modern patient collective. Radiother Oncol July 23–31. https://doi.org/10.1016/j.radonc.2022.04.024
Tamura R, Tanaka T, Miyake K et al (2017) Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol 34:62–77. https://doi.org/10.1007/s10014-017-0284-x
Ginalis EE, Cui T, Weiner J et al (2020) Two-staged stereotactic radiosurgery for the treatment of large brain metastases: single institution experience and review of literature. J Radiosurgery SBRT 7:105–114
Funding
Zhejiang Provincial Department of Education Research Support Project (Y202249332); Zhejiang Provincial Medical and Health Research Support Project (2022KY570); Zhejiang Provincial Medical and Health Research Support Project (2023KY475).
Author information
Authors and Affiliations
Contributions
Wang Zheng, Chen Ninghai, Chen Qun, Zhu Yucun contributed to data collection. Wang Zheng contributed the first manuscript draft and prepared figures and tables. Li Min analyzed all data and made statistics. Zhou Jia supervised the whole project and edited the manuscript. All the authors commented on the previous version of the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no conflicts of interest.
Ethical approval
The present study was approved by the Institutional Ethics Committee of Zhejiang Provincial People’s Hospital (ZHRYRS 2022 No. 005)
Consent to participate and for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Chen, H., Chen, Q. et al. ‘Sandwich treatment’ for posterior fossa brain metastases with volume larger than 4cm3: a multicentric retrospective study. Clin Exp Metastasis 40, 415–422 (2023). https://doi.org/10.1007/s10585-023-10220-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10220-y