Abstract
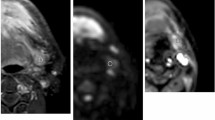
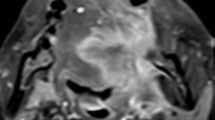
A perfusion defect (PD) in non-enlarged lymph nodes (LNs) of oral squamous cell carcinoma (OSCC) is the most reliable radiological criterion for the diagnosis of metastasis. However, conventional contrast-enhanced (CE) T1 weighted images using turbo spin echo (TSE) sequence is limited in detecting PD in non-enlarged LNs due to flow artifacts from cervical blood vessels. Vessel wall (VW) MR imaging with blood vessel flow suppression and high spatial resolution may provide new insights into the detection of PD. However, there are no reports in the literature on the usefulness of VW MR imaging for the diagnosis of LN metastasis. It is demonstrated that PD of non-enlarged LNs in CE VR MR imaging of OSCC patients is useful for the diagnosis of metastatic LNs. VW MR imaging was significantly more sensitive in detecting PD of non-enlarged metastatic LNs than conventional TSE imaging on visual evaluation. Furthermore, it was found that the image contrast between PD and surrounding intranodal tissue in CE VW MR images was higher than that in conventional CE TSE images. In the correlation between imaging and histopathological findings of metastatic LNs, all LNs that exhibited PD on CE VW MR images were at an advanced histopathological metastatic stage. The pathology of PD was necrotic tissue with keratinization. The results indicated that PD in CE VW imaging is useful in diagnosing non-enlarged LNs at an advanced metastasis stage. The addition of VW MR imaging to conventional MR examination achieves higher diagnostic performance for non-enlarged metastatic LNs.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- PD:
-
Perfusion defect
- LN:
-
Lymph node
- OSCC:
-
Oral squamous cell carcinoma
- MR:
-
Magnetic resonance
- TSE:
-
Turbo spin echo
- CE:
-
Contrast enhanced
- VW:
-
Vessel wall
- MSDE-VISTA:
-
Motion-sensitized driven equilibrium-volume isotropic turbo spin echo acquisition
- CT:
-
Computed tomography
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- T1W:
-
T1-weighted
- T2W:
-
T2-weighted
- SNR:
-
Signal-to-noise ratio
References
Baik SH, Seo JW, Kim JH, Lee SK, Choi EC, Kim J (2019) Prognostic value of cervical nodal necrosis observed in preoperative CT and MRI of patients with tongue squamous cell carcinoma and cervical node metastases: a retrospective study. AJR 213:437–443. https://doi.org/10.2214/ajr.18.20405
van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, Meyer CJ, Snow GB (1990) Cervical lymph node metastasis: assessment of radiographic criteria. Radiology 177:379–384. https://doi.org/10.1148/radiology.177.2.2217772
King AD, Tse GMK, Ahuja AT, Yuen EHY, Vlantis AC, To EWH, van Hasselt AC (2004) Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging and US. Radiology 230:720–726. https://doi.org/10.1148/radiol.2303030157
Morimoto Y, Kurokawa H, Tanaka T, Yamashita Y, Kito S, Okabe S, Takahashi T, Ohba T (2006) Correlation between the incidence of central nodal necrosis in cervical lymph node metastasis and the extent of differentiation in oral squamous cell carcinoma. Dentomaxillofacial Rad 35:18–23. https://doi.org/10.1259/dmfr/24536918
Som PM (1992) Detection of metastasis in cervical lymph nodes: CT and MRI criteria and differential diagnosis. Am J Roentgeno 158:961–969. https://doi.org/10.2214/ajr.158.5.1566697
Yamaki T, Sukhbaatar A, Mishra R, Kikuchi R, Sakamoto M, Mori S, Kodama T (2021) Characterrizing perfusion defects in metastatic lymph nodes at an early stage using high-frequency ultrasound and micro-CT imaging. Clin Exp Metastasis 38:539–549. https://doi.org/10.1007/s10585-021-10127-6
Brown M, Assen FP, Leithner A, Abe J, Scachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M, Kerjaschki D (2018) Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359(6382):1408–1411. https://doi.org/10.1126/science.aal3662
Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EF, Jores D, Chin SM, Kitahara S, Bouta EM, Chang J, Beech E, Jeong HS, Carroll MC, Taghian AG, Padera TP (2018) Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359(6382):1403–1407. https://doi.org/10.1126/science.aal3622
Kodama T, Mori S, Nose M (2018) Tumor cell invasion from the marginal sinus into extranodal veins during early-stage lymph node metastasis can be a starting point for hematogenous metastasis. J Cancer Metastasis Treat 4:56
Takeda K, Mori S, Kodama T (2017) Study of fluid dynamics reveals direct communications between lymphatic vessels and venous blood vessels at lymph nodes of mice. J Immunol Methods 445:1–9
Shao L et al (2015) Communication between lymphatic and venous systems in mice. J Immunol Methods 424:100–105
Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C (2007) Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med 58:973–981
Nagahara S, Nagahara M, Obara M, Kondo R, Minagawa N, Sato S, Sato S, Mouri W, Saito S, Kayama T (2016) Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign ruptured aneurysm? Clin Neuroradiol 26:277–283
Nagano E, Yoshiura T, Hiwatashi A, Obara M, Yamashita K, Kamano H, Takayama Y, Kobayashi K, Honda H (2011) 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MRI imaging. AJNR Am J Neuroradiol 32(4):664–670
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours, 8th edn. Wiley, Chichester, pp 17–21
Curtin HD, Ishwaran H, Mancuso AA, Dalley RW, Caudry DJ, McNeil BJ (1998) Comparison of CT and MR imaging in staging of neck metastasis. Radiology 207:123–130. https://doi.org/10.1148/radiology.207.1.9530307
Friedman M, Robert N, Kirshenbaum GL, Colombo J (1993) Nodal size of metastatic squamous cell carcinoma of the neck. Laryngoscope 103(8):854–856
Tajima Y, Fudano K, Ito K, Aoki T (2013) Fast and robust 3D correspondence matching and its application to volume registration. IEICE Trans Inf and Syst E96-D(4):826–834. https://doi.org/10.1587/transinf.E96.D.826
Don DM, Anzai Y, Lufkin RB, Fu YS, Calcaterra TC (1995) Evaluation of cervical lymph node metastases in squamous cell carcinoma of head and neck. Laryngoscope 105:669–674. https://doi.org/10.1288/00005537-199507000-00001
Chong VF, Fan YF, Khoo JB (1996) MRI features of cervical nodal necrosis in metastatic disease. Clin Radiol 51:103–109. https://doi.org/10.1016/s0009-9260(96)80265-0
Younsem DM, Som PM, Hackney DB, Schwaibold F, Hendrix RA (1992) Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology 182:753–759. https://doi.org/10.1148/radiology.182.3.1535890
Sumi M, Ohki M, Nakamura T (2001) Comparison of sonography and CT for differentiating benign and malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. Am J Roentgenol 176:19–24. https://doi.org/10.2214/ajr.176.4.1761019
Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, Felix R (1995) Cervical lymphadenopathy: ratio of long-to short-axis diameter as a predictor of malignancy. Br J Radiol 68:266–270. https://doi.org/10.1259/0007-1285-68-807-266
Vandecaveye V, De Keyzer F, Poorten VV, Dirix P, Verbeken E, Nuyts S, Hermans R (2009) Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 251:134–146. https://doi.org/10.1148/radiol.2511080128
Abdel Razek KAA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A (2006) Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol 16:1468–1477. https://doi.org/10.1007/s00330-005-0133-x
De Bondt RBJ, Hoeberigs MC, Nelemans PJ, Desero WMLLG, Peutz-Kootstra C, Kremer B, Beets-Tan RGH (2009) Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Head and Neck Radiol 51:183–192. https://doi.org/10.1007/s00234-008-0487-2
Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J (2009) Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol 72:381–387. https://doi.org/10.1016/j.ejrad.2008.09.034
Perrone A, Guerrisi P, Izzo L, D’Angeli I, Sassi S, Lo Mele L, Marini M, Mazza D, Marini M (2011) Diffusion-weighted MRI in cervical lymph nodes: differentiation between benign and malignant lesions. Eur J Radiol 77:281–286. https://doi.org/10.1016/j.ejrad.2009.07.039
Jansen JFA, Stambuk HE, Koutcher JA, Shukla-Dave A (2010) Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: a feasibility study. Am J Neuroradiol 31:741–748. https://doi.org/10.3174/ajnr.A1919
Lim HK, Lee JH, Beak HJ, Kim N, Lee H, Park JW, Kim SY, Cho KJ, Beak JH (2014) Is diffusion-weighted MRI useful for differentiation of small non-necrotic cervical lymph nodes in patients with head and neck malignancies? Korean J Radiol 15:810–816. https://doi.org/10.3348/kjr.2014.15.6.810
Burusapat C, Jarungroongruangchai W, Charoenptakchai M (2015) Prognostic factors of cervical node status in head and neck squamous cell carcinoma. World J of Surg Oncol 13(1):51. https://doi.org/10.1186/s12957-015-0460-6
Broglie MA, Haerle SK, Huber GF, Haile SR, Stoeckli SJ (2013) Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: impact on survival. Head Neck 35:660–666. https://doi.org/10.1002/hed.23017
Acknowledgments
This study was supported in part by JSPS KAKENHI Grants 18H03544 (M.S.), 20H00655 (T.K.), and 21K18319(T.K.). We thank Hideki Ota, Tatsuo Nagasaka, and Ariunbuyan Sukhbaatar for their excellent technical support. In addition, we thank Mika Watanabe for her histopathological support.
Author information
Authors and Affiliations
Contributions
SM interpreted all of the data and supervised the project. KI and IM performed the imaging analyses and statistical analysis. IK and AT analyzed the imaging data using a developed proprietary volume registration method. OT, KY and MS performed neck dissection and the pathological analysis. ID and MT designed the new magnetic resonance imaging methodologies and interpreted the imaging data. KT interpreted all of the data and supervised the project.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakamoto, M., Kojima, I., Iikubo, M. et al. Perfusion defects in non-enlarged metastatic lymph nodes using vessel wall magnetic resonance imaging: Detection performance and diagnostic value. Clin Exp Metastasis 39, 421–431 (2022). https://doi.org/10.1007/s10585-022-10147-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-022-10147-w