“Far, far beneath in the abysmal sea, The Kraken sleepeth.
Until the latter fire shall heat the deep; In roaring he shall rise.” Alfred Lord Tennyson
Abstract
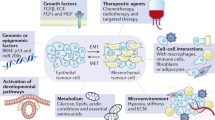
Epithelial mesenchymal transition (EMT) describes the shift of cells from an epithelial form to a contact independent, migratory, mesenchymal form. In cancer the change is linked to invasion and metastasis. Tumour conditions, including hypoxia, acidosis and a range of treatments can trigger EMT, which is implicated in the subsequent development of resistance to those same treatments. Consequently, the degree to which EMT occurs may underpin the entire course of tumour progression and treatment response in a patient. In this review we look past the protective effect of EMT against the initial treatment, to the role of the mesenchymal state, once triggered, in promoting disease growth, spread and future treatment insensitivity. In patients a correlation was found between the propensity of a treatment to induce EMT and failure of that treatment to provide a survival benefit, implicating EMT induction in accelerated tumour progression after treatment cessation. Looking to the mechanisms driving this detrimental effect; increased proliferation, suppressed apoptosis, stem cell induction, augmented angiogenesis, enhanced metastatic dissemination, and immune tolerance, can all result from treatment-induced EMT and could worsen outcome. Evidence also suggests EMT induction with earlier therapies attenuates benefits of later treatments. Looking beyond epithelial tumours, de-differentiation also has therapy-attenuating effects and reversal thereof may yield similar rewards. A range of potential therapies are in development that may address the diverse mechanisms and molecular control systems involved in EMT-induced accelerated progression. Considering the broad reaching effects of mesenchymal shift identified, successful deployment of such treatments could substantially improve patient outcomes.


Similar content being viewed by others
Abbreviations
- ALDH1:
-
Aldehyde dehydrogenase 1
- ALK:
-
Anaplastic lymphoma kinase
- ATRA:
-
All-trans retinoic acid
- CCL2:
-
Chemokine ligand 2
- CRISPR:
-
Clustered regulatory interspaced short palindromic repeats
- CSCs:
-
Cancer stem cells
- CTCs:
-
Circulating tumour cells
- CTLA-4:
-
Cytotoxic T-lymphocyte–associated antigen 4
- EGF:
-
Epidermal growth factor
- EIS:
-
EMT-inhibiting sextet
- EM-axis:
-
Epithelial-mesenchymal axis
- EMP:
-
Epithelial-mesenchymal plasticity
- EMT:
-
Epithelial mesenchymal transition
- EMT-TFs:
-
EMT-transcriptional factors
- ERα:
-
Estrogen receptor alpha
- ERK:
-
Extracellular signal-regulated kinase
- HCC:
-
Hepatocellular carcinoma
- HDAC:
-
Histone deacetylase
- HER2:
-
Human epidermal growth factor receptor 2
- HIF1α:
-
Hypoxia inducible factor-1α
- IAP:
-
Inhibitor of apoptosis
- MAPK:
-
Mitogen activated protein kinase
- MEK:
-
Mitogen-activated protein/extracellular signal-regulated kinase kinase
- MET:
-
Mesenchymal epithelial transition
- miRNA:
-
MicroRNA
- MMP:
-
Matrix metalloproteinase
- NF-kB:
-
Nuclear factor kB
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD1:
-
Programmed death 1
- PD-L1:
-
Programmed death ligand 1
- PFS:
-
Progression-free survival
- TGF-β:
-
Transforming growth factor beta
- VEGF:
-
Vascular endothelial growth factor
References
Halbleib J, Nelson WJ (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20(23):3199–3214
Kawano S, Asano M, Adachi Y, Matsui J (2016) Antimitotic and non-mitotic effects of eribulin mesilate in soft tissue sarcoma. Anticancer Res 36(4):1553–1561
Ekblom P (1989) Developmentally regulated conversion of mesenchyme to epithelium. FASEB J 3(10):2141–2150
Haensel D, Dai X (2018) Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev Dyn. https://doi.org/10.1002/dvdy.24561
Tomaskovic-Crook E, Thompson EW, Thiery JP (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11(6):213. https://doi.org/10.1186/bcr2416
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. https://doi.org/10.1016/j.cell.2009.11.007
Tan T, Miow Q, Miki Y, Noda T, Mori S, Huang R, Thiery J (2014) Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 6(10):1279–1293
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J (2008) Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68(4):989–997
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12(5):R68. https://doi.org/10.1186/bcr2635
McInnes LM, Jacobson N, Redfern A, Dowling A, Thompson EW, Saunders CM (2015) Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol 5:42. https://doi.org/10.3389/fonc.2015.00042
Raimondi C, Gradilone A, Naso G, Vincenzi B, Petracca A, Nicolazzo C, Palazzo A, Saltarelli R, Spremberg F, Cortesi E, Gazzaniga P (2011) Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat 130(2):449–455. https://doi.org/10.1007/s10549-011-1373-x
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339(6119):580–584. https://doi.org/10.1126/science.1228522
Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW (2010) Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 15(2):235–252. https://doi.org/10.1007/s10911-010-9175-z
Kotiyal S, Bhattacharya S (2014) Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun 453(1):112–116. https://doi.org/10.1016/j.bbrc.2014.09.069
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3(8):e2888
Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 14(10):611–629. https://doi.org/10.1038/nrclinonc.2017.44
Cao Z, Livas T, Kyprianou N (2016) Anoikis and EMT: lethal “liaisons” during cancer progression. Critical reviews in oncogenesis 21(3–4):155–168
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:179
Thompson EW, Haviv I (2011) The social aspects of EMT-MET plasticity. Nat Med 17(9):1048–1049. https://doi.org/10.1038/nm.2437
Gunasinghe NPAD., Wells A, Thompson E, Hugo H (2012) Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev 31(3–4):469–478
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J (2012) Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22(6):725–736. https://doi.org/10.1016/j.ccr.2012.09.022
Ocaña Oscar H, Córcoles R, Fabra Á, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA (2012) Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22(6):709–724. https://doi.org/10.1016/j.ccr.2012.10.012
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y (2011) Direct targeting of Sec23a by miR-200 s influences cancer cell secretome and promotes metastatic colonization. Nature Med 17(9):1101–1108. https://doi.org/10.1038/nm.2401
Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527(7579):472–476. https://doi.org/10.1038/nature15748
Krebs A, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton V, Pilarsky C, Winkler T, Brabletz S, Stemmler M, Brabletz T (2017) The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 19(5):518–529
Ye X, Brabletz T, Kang Y, Longmore GD, Nieto MA, Stanger BZ, Yang J, Weinberg RA (2017) Upholding a role for EMT in breast cancer metastasis. Nature 547:E1. https://doi.org/10.1038/nature22816
Stankic M, Pavlovic S, Chin Y, Brogi E, Padua D, Norton L, Massague J, Benezra R (2013) TGFβ-Id1 Signaling opposes twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep 5(5):1228–1242. https://doi.org/10.1016/j.celrep.2013.11.014
Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktas M, Hwang MS, Darling DS, Coleman IM, Nelson PS, Nguyen HM, Corey E, Tewari M, Morrissey C, Vessella RL, Knudsen BS (2011) Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol 179(1):400–410. https://doi.org/10.1016/j.ajpath.2011.03.028
Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, Onuchic JN, Levine H (2015) Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol 5:155. https://doi.org/10.3389/fonc.2015.00155
Lee JM, Dedhar S, Kalluri R, Thompson EW (2006) The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172(7):973–981. https://doi.org/10.1083/jcb.200601018
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijón E, Balsat C, Sokolow Y, Dubois C, De Cock F, Scozzaro S, Sopena F, Lanas A, D’Haene N, Salmon I, Marine J-C, Voet T, Sotiropoulou P, Blanpain C (2018) Identification of the tumour transition states occurring during EMT. Nature 556(7702):463–468
Beerling E, Seinstra D, de Wit E, Kester L, van der Velden D, Maynard C, Schäfer R, van Diest P, Voest E, van Oudenaarden A, Vrisekoop N, van Rheenen J (2016) Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep 14(10):2281–2288. https://doi.org/10.1016/j.celrep.2016.02.034
Celià-Terrassa T, Meca-Cortés Ó, Mateo F, Martínez de Paz A, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra-Rebollo M, Lozano JJ, Estarás C, Ulloa C, ρlvarez-Simón D, Milà J, Vilella R, Paciucci R, Martínez-Balbás M, García de Herreros A, Gomis RR, Kang Y, Blanco J, Fernández PL, Thomson TM (2012) Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Investig 122(5):1849–1868. https://doi.org/10.1172/JCI59218
Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu G-f (2008) Epithelial-mesenchymal transition induced by growth suppressor p12(CDK2-AP1) promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 68(24):10377–10386. https://doi.org/10.1158/0008-5472.CAN-08-1444
Fidler IJ (1973) The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9(3):223–227
Alix-Panabières C, Mader S, Pantel K (2017) Epithelial-mesenchymal plasticity in circulating tumor cells. J Mol Med 95(2):133–142. https://doi.org/10.1007/s00109-016-1500-6
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17(5):548–558. https://doi.org/10.1016/j.ceb.2005.08.001
Puisieux A, Brabletz T, Caramel J (2014) Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 16(6):488–494. https://doi.org/10.1038/ncb2976
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119(6):1429–1437. https://doi.org/10.1172/JCI36183
Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY-J, Thiery JP (2014) Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 6(10):1279–1293. https://doi.org/10.15252/emmm.201404208
Foroutan M, Bhuva D, Horan K, Lyu R, Cursons J, Davis MJ (2017) Defining a landscape of molecular phenotypes using a simple single sample scoring method. bioRxiv. https://doi.org/10.1101/231217
Cursons J, Leuchowius K-J, Waltham M, Tomaskovic-Crook E, Foroutan M, Bracken CP, Redfern A, Crampin EJ, Street I, Davis MJ, Thompson EW (2015) Stimulus-dependent differences in signalling regulate epithelial-mesenchymal plasticity and change the effects of drugs in breast cancer cell lines. Cell Commun Signal 13:26. https://doi.org/10.1186/s12964-015-0106-x
Peinado H, Ballestar E, Esteller M, Cano A (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24(1):306–319. https://doi.org/10.1128/MCB.24.1.306-319.2004
Lin T, Ponn A, Hu X, Law BK, Lu J (2010) Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene 29(35):4896–4904. https://doi.org/10.1038/onc.2010.234
Nagathihalli N, Massion P, Gonzalez A, Lu P, Datta P (2012) Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol Cancer Ther 11(11):2362–2372
Yang F, Sun L, Li Q, Han X, Lei L, Zhang H, Shang Y (2012) SET8 promotes epithelial–mesenchymal transition and confers TWIST dual transcriptional activities. EMBO J 31(1):110–123. https://doi.org/10.1038/emboj.2011.364
Yang M-H, Hsu D, Wang H-J, Lan H-Y, Yang W-H, Huang C-H, Kao S-Y, Tzeng C-H, Tai S-K, Chang S-Y, Lee O, Wu K-J (2010) Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 12(10):982–992
Sánchez Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A (2010) ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 29(24):3490–3500
Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn C-O, Heidecke C-D, Friess H, Büchler M, Evert M, Lerch M, Weiss F (2012) Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 61(3):439–448
Liao W, Jordaan G, Srivastava MK, Dubinett S, Sharma S, Sharma S (2013) Effect of epigenetic histone modifications on E-cadherin splicing and expression in lung cancer. Am J Cancer Res 3(4):374–389
Sharma S, Lichtenstein A (2009) Aberrant splicing of the E-cadherin transcript is a novel mechanism of gene silencing in chronic lymphocytic leukemia cells. Blood 114(19):4179–4185
Jordaan G, Liao W, Sharma S (2013) E-cadherin gene re-expression in chronic lymphocytic leukemia cells by HDAC inhibitors. BMC Cancer 13(1):88. https://doi.org/10.1186/1471-2407-13-88
Sakamoto T, Kobayashi S, Yamada D, Nagano H, Tomokuni A, Tomimaru Y, Noda T, Gotoh K, Asaoka T, Wada H, Kawamoto K, Marubashi S, Eguchi H, Doki Y, Mori M (2016) A histone deacetylase inhibitor suppresses epithelial-mesenchymal transition and attenuates chemoresistance in biliary tract cancer. PLoS ONE 11(1):e0145985. https://doi.org/10.1371/journal.pone.0145985
Rhodes LV, Tate CR, Segar HC, Burks HE, Phamduy TB, Hoang V, Elliott S, Gilliam D, Pounder FN, Anbalagan M, Chrisey DB, Rowan BG, Burow ME, Collins-Burow BM (2014) Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res Treat 145(3):593–604. https://doi.org/10.1007/s10549-014-2979-6
Ruscetti M, Dadashian EL, Guo W, Quach B, Mulholland DJ, Park JW, Tran LM, Kobayashi N, Bianchi-Frias D, Xing Y, Nelson PS, Wu H (2016) HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 35(29):3781–3795. https://doi.org/10.1038/onc.2015.444
Giudice FS, Pinto DS, Nör JE, Squarize CH, Castilho RM (2013) Inhibition of histone deacetylase impacts cancer stem cells and induces epithelial-mesenchyme transition of head and neck cancer. PLoS ONE 8(3):e58672. https://doi.org/10.1371/journal.pone.0058672
Feng J, Cen J, Li J, Zhao R, Zhu C, Wang Z, Xie J, Tang W (2015) Histone deacetylase inhibitor valproic acid (VPA) promotes the epithelial mesenchymal transition of colorectal cancer cells via up regulation of Snail. Cell Adhes Migr 9(6):495–501. https://doi.org/10.1080/19336918.2015.1112486
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119(6):1420–1428. https://doi.org/10.1172/JCI39104
Zhu Q-C, Gao R-Y, Wu W, Qin H-L (2013) Epithelial-mesenchymal transition and its role in the pathogenesis of colorectal cancer. Asian Pac J Cancer Prev 14(5):2689–2698
Xu S, Chheda C, Ouhaddi Y, Benhaddou H, Bourhim M, Grippo P, Principe D, Mascariñas E, DeCant B, Tsukamoto H, Pandol S, Edderkaoui M (2015) Characterization of mouse models of early pancreatic lesions induced by alcohol and chronic pancreatitis. Pancreas 44(6):882–887
Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö (2017) Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, porphyromonas gingivalis. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00493
Yang S, Da Zhang L, Xiong Y, Zhang Y, Li H, Li X, Dong J (2009) HBx protein induces EMT through c-Src activation in SMMC-7721 hepatoma cell line. Biochem Biophys Res Commun 382(3):555–560
Shen H-J, Sun Y-H, Zhang S-J, Jiang J-X, Dong X-W, Jia Y-L, Shen J, Guan Y, Zhang L-H, Li F-F, Lin X-X, Wu X-M, Xie Q-M, Yan X-F (2014) Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim Biophys Acta 1840 (6):1838–1849
Wang Y, Xu M, Ke Z-J, Luo J (2017) Cellular and molecular mechanism underlying alcohol-induced aggressiveness of breast cancer. Pharmacol Res 115:299–308. https://doi.org/10.1016/j.phrs.2016.12.005
Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L, Dou C, Liu Q, Zheng X (2017) Hypoxia accelerates aggressiveness of hepatocellular carcinoma cells involving oxidative stress, epithelial-mesenchymal transition and non-canonical hedgehog signaling. Cell Physiol Biochem 44(5):1856–1868
Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. https://doi.org/10.1016/j.ccr.2011.11.024
Steeg PS, Anderson RL, Bar-Eli M, Chambers AF, Eccles SA, Hunter K, Itoh K, Kang Y, Matrisian LM, Sleeman JP, Theodorescu D, Thompson EW, Welch DR (2009) An open letter to the FDA and other regulatory agencies: preclinical drug development must consider the impact on metastasis. Clin Cancer Res 15:4529–4529. https://doi.org/10.1158/1078-0432.CCR-09-1363
Peppicelli S, Bianchini F, Torre E, Calorini L (2014) Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin Exp Metastasis 31(4):423–433
Deng S, Li X, Niu Y, Zhu S, Jin Y, Chen J, Liu Y, He C, Yin T, Yang Z, Tao J, Xiong J, Wu H, Wang C, Zhao G (2015) MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1. Oncotarget 6(37):39661–39675
Suzuki A, Maeda T, Baba Y, Shimamura K, Kato Y (2014) Acidic extracellular pH promotes epithelial mesenchymal transition in Lewis lung carcinoma model. Cancer Cell Int 14(1):129–129
Marín-Hernández Á, Gallardo-Pérez JC, Hernández Reséndiz I, Del Mazo-Monsalvo I, Robledo-Cadena DX, Moreno Sánchez R, Rodríguez Enríquez S (2017) Hypoglycemia enhances epithelial-mesenchymal transition and invasiveness, and restrains the warburg phenotype, in hypoxic HeLa cell cultures and microspheroids. J Cell Physiol 232(6):1346–1359
Aoyagi K, Tamaoki M, Nishumura T, Sasaki H (2013) Technical considerations for analyzing EMT-MET data from surgical samples. Cancer Lett 341(1):105–110. https://doi.org/10.1016/j.canlet.2013.08.001
Saxena M, Stephens MA, Pathak H, Rangarajan A (2011) Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis 2:e179. https://doi.org/10.1038/cddis.2011.61
Krohn A, Ahrens T, Yalcin A, Plones T, Wehrle J, Taromi S, Wollner S, Follo M, Brabletz T, Mani SA, Claus R, Hackanson B, Burger M (2014) Tumor cell heterogeneity in Small Cell Lung Cancer (SCLC): phenotypical and functional differences associated with Epithelial-Mesenchymal Transition (EMT) and DNA methylation changes. PLoS ONE 9(6):e100249. https://doi.org/10.1371/journal.pone.0100249
Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H (2012) EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 12:91–91. https://doi.org/10.1186/1471-2407-12-91
Yan Y-R, Xie Q, Li F, Zhang Y, Ma J-W, Xie S-M, Li H-Y, Zhong X-Y (2014) Epithelial-to-mesenchymal transition is involved in BCNU resistance in human glioma cells. Neuropathology 34(2):128–134
Xiong H, Hong J, Du W, Lin Y-W, Ren L-L, Wang Y-C, Su W-Y, Wang J-L, Cui Y, Wang Z-H, Fang J-Y (2012) Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem 287(8):5819–5832
Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH (2009) Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res 69(16):6704–6712. https://doi.org/10.1158/0008-5472.CAN-09-1298
Zhang G, Tian X, Li Y, Wang Z, Li X, Zhu C (2018) miR-27b and miR-34a enhance docetaxel sensitivity of prostate cancer cells through inhibiting epithelial-to-mesenchymal transition by targeting ZEB1. Biomed Pharmacother 97:736–744. https://doi.org/10.1016/j.biopha.2017.10.163
Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R, Barrow D, Nicholson RI (2006) Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer 118(2):290–301. https://doi.org/10.1002/ijc.21355
Huang S, Liu Q, Liao Q, Wu Q, Sun B, Yang Z, Hu X, Tan M, Li L (2018) IL-6/STAT3 promotes prostate cancer resistance to androgen deprivation therapy via regulating PTTG1 expression. Cancer Sci. https://doi.org/10.1111/cas.13493
Lesniak D, Sabri S, Xu Y, Graham K, Bhatnagar P, Suresh M, Abdulkarim B (2013) Spontaneous epithelial-mesenchymal transition and resistance to HER-2-targeted therapies in HER-2-positive luminal breast cancer. PLoS ONE 8(8):e71987. https://doi.org/10.1371/journal.pone.0071987
Yang Z, Guo L, Liu D, Sun L, Chen H, Deng Q, Liu Y, Yu M, Ma Y, Guo N, Shi M (2015) Acquisition of resistance to trastuzumab in gastric cancer cells is associated with activation of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget 6(7):5072–5087
Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA Jr, Raben D (2007) Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther 6(6):1683–1691. https://doi.org/10.1158/1535-7163.MCT-07-0138
Kim HR, Kim WS, Choi YJ, Choi CM, Rho Jin K, Lee JC (2013) Epithelial-mesenchymal transition leads to crizotinib resistance in H2228 lung cancer cells with EML4-ALK translocation. Mol Oncol 7(6):1093–1102. https://doi.org/10.1016/j.molonc.2013.08.001
Ramsdale R, Jorissen R, Li F, Al Obaidi S, Ward T, Sheppard K, Bukczynska P, Young R, Boyle S, Shackleton M, Bollag G, Long G, Tulchinsky E, Rizos H, Pearson R, McArthur G, Dhillon A, Ferrao P (2015) The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci Signal 8(390):ra82
Miyazaki S, Kikuchi H, Iino I, Uehara T, Setoguchi T, Fujita T, Hiramatsu Y, Ohta M, Kamiya K, Kitagawa K, Kitagawa M, Baba S, Konno H (2014) Anti-VEGF antibody therapy induces tumor hypoxia and stanniocalcin 2 expression and potentiates growth of human colon cancer xenografts. Int J Cancer 135(2):295–307
Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K, Matsumoto T, Matsuura N, Ohta M, Okumura M (2011) Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg 92(5):1794–1804. (discussion 1804)
Marin-Aguilera M, Codony-Servat J, Reig O, Lozano JJ, Fernandez PL, Pereira MV, Jimenez N, Donovan M, Puig P, Mengual L, Bermudo R, Font A, Gallardo E, Ribal MJ, Alcaraz A, Gascon P, Mellado B (2014) Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol Cancer Ther 13(5):1270–1284. https://doi.org/10.1158/1535-7163.MCT-13-0775
Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K, Matsumoto T, Matsuura N, Ohta M, Okumura M (2011) Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg 92(5):1794–1804. https://doi.org/10.1016/j.athoracsur.2011.07.032. (discussion 1804)
Redfern AD, McLaren SA, Dissanayake V, Chan A, Zeps N, Dobrovic A, Soon L, Thompson EW, Christobel SM (2016) Predictive value of de novo and induced epithelial-mesenchymal transition in locally advanced breast cancer treated with neoadjuvant chemotherapy. Cancer Res 76(4 Suppl):P1-05-03
Hara J, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y (2014) Mesenchymal phenotype after chemotherapy is associated with chemoresistance and poor clinical outcome in esophageal cancer. Oncol Rep 31(2):589–596. https://doi.org/10.3892/or.2013.2876
Kawamoto A, Yokoe T, Tanaka K, Saigusa S, Toiyama Y, Yasuda H, Inoue Y, Miki C, Kusunoki M (2012) Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol Rep 27(1):51–57. https://doi.org/10.3892/or.2011.1485
Fifis T, Nguyen L, Malcontenti Wilson C, Chan L, Nunes Costa P, Daruwalla J, Nikfarjam M, Muralidharan V, Waltham M, Thompson E, Christophi C (2013) Treatment with the vascular disruptive agent OXi4503 induces an immediate and widespread epithelial to mesenchymal transition in the surviving tumor. Cancer Med 2(5):595–610
Nörz D, Grottke A, Bach J, Herzberger C, Hofmann B, Nashan B, Jücker M, Ewald F (2015) Discontinuing MEK inhibitors in tumor cells with an acquired resistance increases migration and invasion. Cell Signal 27(11):2191–2200
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, Armitage GR, Wilson JJ, Venner PM, Coppin CM, Murphy KC (1996) Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 14(6):1756–1764. https://doi.org/10.1200/jco.1996.14.6.1756
Li C, Xiang A, Chen X, Yin K, Lu J, Yin W (2017) Optimizing the treatment of bevacizumab as first-line therapy for human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer: an updated meta-analysis of published randomized trials. OncoTargets Ther 10:3155–3168. https://doi.org/10.2147/OTT.S138600
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 106(33):13820–13825. https://doi.org/10.1073/pnas.0905718106
Martín M, Loibl S, von Minckwitz G, Morales S, Martinez N, Guerrero A, Anton A, Aktas B, Schoenegg W, Muñoz M, Garcia-Saenz J, Gil M, Ramos M, Margeli M, Carrasco E, Liedtke C, Wachsmann G, Mehta K, De la Haba-Rodriguez JR (2015) Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol 33(9):1045–1052
Jayachandran A, Anaka M, Prithviraj P, Hudson C, McKeown SJ, Lo P-H, Vella LJ, Goding CR, Cebon J, Behren A (2014) Thrombospondin 1 promotes an aggressive phenotype through epithelial-to-mesenchymal transition in human melanoma. Oncotarget 5(14):5782–5797
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, Garbe C, Testori A, Maio M, Lorigan P, Lebbé C, Jouary T, Schadendorf D, O’Day SJ, Kirkwood JM, Eggermont AM, Dréno B, Sosman JA, Flaherty KT, Yin M, Caro I, Cheng S, Trunzer K, Hauschild A (2014) Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 15(3):323–332. https://doi.org/10.1016/S1470-2045(14)70012-9
de Bono J, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, Gravis G, Bodrogi I, Mackenzie M, Shen L, Roessner M, Gupta S, Sartor AO (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376(9747):1147–1154
Martin SK, Pu H, Penticuff JC, Cao Z, Horbinski C, Kyprianou N (2016) Multinucleation and mesenchymal-to-epithelial-transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res 76(4):912–926. https://doi.org/10.1158/0008-5472.CAN-15-2078
Yardley DA, Noguchi S, Pritchard KI, Burris HA, Baselga J, Gnant M, Hortobagyi GN, Campone M, Pistilli B, Piccart M, Melichar B, Petrakova K, Arena FP, Erdkamp F, Harb WA, Feng W, Cahana A, Taran T, Lebwohl D, Rugo HS (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30(10):870–884. https://doi.org/10.1007/s12325-013-0060-1
Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, Noguchi S, Perez A, Rugo HS, Deleu I, Burris HA, Provencher L, Neven P, Gnant M, Shtivelband M, Wu C, Fan J, Feng W, Taran T, Baselga J (2014) Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 25(12):2357–2362
Terashima M, Sakai K, Togashi Y, Hayashi H, De Velasco MA, Tsurutani J, Nishio K (2014) Synergistic antitumor effects of S-1 with eribulin in vitro and in vivo for triple-negative breast cancer cell lines. SpringerPlus 3:417. https://doi.org/10.1186/2193-1801-3-417
Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman P, Awada A (2014) Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat 148(3):553–561
Schöffski P, Chawla S, Maki R, Italiano A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha S, Blay J-Y, Hohenberger P, D’Adamo D, Guo M, Chmielowski B, Le Cesne A, Demetri G, Patel S (2016) Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 387(10028):1629–1637
Demetri G, Schöffski P, Grignani G, Blay J-Y, Maki R, Van Tine B, Alcindor T, Jones R, D’Adamo D, Guo M, Chawla S (2017) Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J Clin Oncol 35(30):3433–3439
Shah P, Gau Y, Sabnis G (2014) Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 143(1):99–111
Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee MJ, Trepel JB (2013) Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31(17):2128–2135. https://doi.org/10.1200/JCO.2012.43.7251
Hugo HJ, Pereira L, Suryadinata R, Drabsch Y, Gonda TJ, Gunasinghe NPAD., Pinto C, Soo ETL, van Denderen BJW, Hill P, Ramsay RG, Sarcevic B, Newgreen DF, Thompson EW (2013) Direct repression of MYB by ZEB1 suppresses proliferation and epithelial gene expression during epithelial-to-mesenchymal transition of breast cancer cells. Breast Cancer Res 15(6):R113-R113. https://doi.org/10.1186/bcr3580
Hugo HJ, Gunasinghe NPAD., Hollier BG, Tanaka T, Blick T, Toh A, Hill P, Gilles C, Waltham M, Thompson EW (2017) Epithelial requirement for in vitro proliferation and xenograft growth and metastasis of MDA-MB-468 human breast cancer cells: oncogenic rather than tumor-suppressive role of E-cadherin. Breast Cancer Res 19:86. https://doi.org/10.1186/s13058-017-0880-z
Li J, Yang S, Yan W, Yang J, Qin Y-J, Lin X-L, Xie R-Y, Wang S-C, Jin W, Gao F, Shi J-W, Zhao W-T, Jia J-S, Shen H-F, Ke J-R, Liu B, Zhao Y-Q, Huang W-H, Yao K-T, Li D-J, Xiao D (2015) MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Invest 95(9):1056–1070
Bouris P, Skandalis SS, Piperigkou Z, Afratis N, Karamanou K, Aletras AJ, Moustakas A, Theocharis AD, Karamanos NK (2015) Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol 43:42–60. https://doi.org/10.1016/j.matbio.2015.02.008
Rosanò L, Cianfrocca R, Spinella F, Di Castro V, Nicotra M, Lucidi A, Ferrandina G, Natali P, Bagnato A (2011) Acquisition of chemoresistance and EMT phenotype is linked with activation of the endothelin A receptor pathway in ovarian carcinoma cells. Clin Cancer Res 17(8):2350–2360
Sánchez Tilló E, Fanlo L, Siles L, Montes Moreno S, Moros A, Chiva Blanch G, Estruch R, Martinez A, Colomer D, Győrffy B, Roué G, Postigo A (2014) The EMT activator ZEB1 promotes tumor growth and determines differential response to chemotherapy in mantle cell lymphoma. Cell Death Differ 21(2):247–257
Sun Y, Guan Z, Liang L, Cheng Y, Zhou J, Li J, Xu Y (2016) NF-κB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. Int J Oncol 48(1):225–234
Willipinski Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, Heukeshoven J, Pantel K (2005) Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 11(22):8006–8014
Seshadri R, Raymond WA, Leong AS, Horsfall DJ, McCaul K (1996) Vimentin expression is not associated with poor prognosis in breast cancer. Int J Cancer 67(3):353–356
Montserrat N, Gallardo A, Escuin D, Catasus L, Prat J, Gutiérrez Avignó F, Peiró G, Barnadas A, Lerma E (2011) Repression of E-cadherin by SNAIL, ZEB1, and TWIST in invasive ductal carcinomas of the breast: a cooperative effort? Hum Pathol 42(1):103–110
Withers HR, Taylor JM, Maciejewski B (1988) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27(2):131–146
Huang Z, Mayr NA, Gao M, Lo SS, Wang JZ, Jia G, Yuh WTC (2012) The onset time of tumor repopulation for cervical cancer—first evidence from clinical data. Int J Radiat Oncol Biol Phys 84(2):478–484. https://doi.org/10.1016/j.ijrobp.2011.12.037
Maciejewski B, Majewski S (1991) Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol 21(3):163–170
Stephens TC, Peacock JH (1977) Tumour volume response, initial cell kill and cellular repopulation in B16 melanoma treated with cyclophosphamide and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Br J Cancer 36(3):313–321
Wu L, Tannock I (2003) Repopulation in murine breast tumors during and after sequential treatments with cyclophosphamide and 5-fluorouracil. Cancer Res 63(9):2134–2138
Wu C-T, Chen W-C, Liao S-K, Hsu C-L, Lee K-D, Chen M-F (2007) The radiation response of hormone-resistant prostate cancer induced by long-term hormone therapy. Endocr Relat Cancer 14(3):633–643
Bourhis J, Wilson G, Wibault P, Janot F, Bosq J, Armand JP, Luboinski B, Malaise EP, Eschwege F (1994) Rapid tumor cell proliferation after induction chemotherapy in oropharyngeal cancer. Laryngoscope 104(4):468–472
Kajita M, McClinic KN, Wade PA (2004) Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol 24(17):7559–7566. https://doi.org/10.1128/MCB.24.17.7559-7566.2004
Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL (2011) TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 71(13):4707–4719. https://doi.org/10.1158/0008-5472.CAN-10-4554
Mikami S, Mizuno R, Kosaka T, Saya H, Oya M, Okada Y (2015) Expression of TNF-α and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int J Cancer 136(7):1504–1514
Lombardo Y, Faronato M, Filipovic A, Vircillo V, Magnani L, Coombes RC (2014) Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Res 16(3):R62-R62. https://doi.org/10.1186/bcr3675
Moore N, Lyle S (2011) Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol 2011:396076. https://doi.org/10.1155/2011/396076
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, Hong S, Adams A, D’Angelo R, Ginestier C, Charafe-Jauffret E, Clouthier SG, Birnbaum D, Wong ST, Zhan M, Chang JC, Wicha MS (2014) Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep 2(1):78–91. https://doi.org/10.1016/j.stemcr.2013.11.009
Lagadec C, Vlashi E, Della Donna L, Meng Y, Dekmezian C, Kim K, Pajonk F (2010) Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res 12(1):R13. https://doi.org/10.1186/bcr2479
Bao S, Wu Q, McLendon R, Hao Y, Shi Q, Hjelmeland A, Dewhirst M, Bigner D, Rich J (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):756–760
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133(+)cancer stem cells in glioblastoma. Mol Cancer 5:67–67. https://doi.org/10.1186/1476-4598-5-67
Bourguignon LYW, Wong G, Earle C, Chen L (2012) Hyaluronan-CD44v3 interaction with Oct4-Sox2-nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 287(39):32800–32824. https://doi.org/10.1074/jbc.M111.308528
Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J (2013) Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res 73(2):662–671. https://doi.org/10.1158/0008-5472.CAN-12-0653
Gu A, Jie Y, Yao Q, Zhang Y, Mingyan E (2017) Slug is associated with tumor metastasis and angiogenesis in ovarian cancer. Reprod Sci 24(2):291–299
Zhang Y, Liu Y, Zou J, Yan L, Du W, Zhang Y, Sun H, Lu P, Geng S, Gu R, Zhang H, Bi Z (2017) Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1α and autophagy in human osteosarcoma. Oncotarget 8(53):91134–91149. https://doi.org/10.18632/oncotarget.19845
Xu H, Rahimpour S, Nesvick CL, Zhang X, Ma J, Zhang M, Zhang G, Wang L, Yang C, Hong CS, Germanwala AV, Elder JB, Ray-Chaudhury A, Yao Y, Gilbert MR, Lonser RR, Heiss JD, Brady RO, Mao Y, Qin J, Zhuang Z (2015) Activation of hypoxia signaling induces phenotypic transformation of glioma cells: implications for bevacizumab antiangiogenic therapy. Oncotarget 6(14):11882–11893
Luo J, Lubaroff DM, Hendrix MJ (1999) Suppression of prostate cancer invasive potential and matrix metalloproteinase activity by E-cadherin transfection. Cancer Res 59(15):3552–3556
Llorens A, Rodrigo I, López Barcons L, Gonzalez Garrigues M, Lozano E, Vinyals A, Quintanilla M, Cano A, Fabra A (1998) Down-regulation of E-cadherin in mouse skin carcinoma cells enhances a migratory and invasive phenotype linked to matrix metalloproteinase-9 gelatinase expression. Lab Invest 78(9):1131–1142
Ungefroren H, Witte D, Lehnert H (2017) The role of small GTPases of the Rho/Rac family in TGF-beta-induced EMT and cell motility in cancer. Dev Dyn. https://doi.org/10.1002/dvdy.24505
Yang W-H, Lan H-Y, Huang C-H, Tai S-K, Tzeng C-H, Kao S-Y, Wu K-J, Hung M-C, Yang M-H (2012) RAC1 activation mediates Twist1-induced cancer cell migration. Nat Cell Biol 14(4):366–374
Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grünert S (2002) Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 156(2):299–314. https://doi.org/10.1083/jcb.200109037
Kudo Saito C, Shirako H, Takeuchi T, Kawakami Y (2009) Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15(3):195–206
Muraoka Cook R, Kurokawa H, Koh Y, Forbes J, Roebuck LR, Barcellos Hoff M, Moody S, Chodosh L, Arteaga C (2004) Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res 64(24):9002–9011
Kakeji Y, Maehara Y, Ikebe M, Teicher BA (1997) Dynamics of tumor oxygenation, CD31 staining and transforming growth factor-beta levels after treatment with radiation or cyclophosphamide in the rat 13762 mammary carcinoma. Int J Radiat Oncol Biol Phys 37(5):1115–1123
Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA (1994) Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Investig 93(2):892–899
Skowron MA, Niegisch G, Fritz G, Arent T, van Roermund JGH, Romano A, Albers P, Schulz WA, Hoffmann MJ (2015) Phenotype plasticity rather than repopulation from CD90/CK14 + cancer stem cells leads to cisplatin resistance of urothelial carcinoma cell lines. J Exp Clinical Cancer Res 34:144. https://doi.org/10.1186/s13046-015-0259-x
DiMeo TA, Anderson K, Phadke P, Feng C, Perou CM, Naber S, Kuperwasser C (2009) A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res 69(13):5364–5373. https://doi.org/10.1158/0008-5472.CAN-08-4135
Piva MBR, Jakubzig B, Bendas G (2017) Integrin activation contributes to lower cisplatin sensitivity in MV3 melanoma cells by inducing the wnt signalling pathway. Cancers 9(9):125. https://doi.org/10.3390/cancers9090125
Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE (2013) The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer 13:174–174. https://doi.org/10.1186/1471-2407-13-174
van Putten LM, Kram LK, van Dierendonck HH, Smink T, Füzy M (1975) Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer 15(4):588–595
Vollmer TL, Conley FK (1984) Effect of cyclophosphamide on survival of mice and incidence of metastatic tumor following intravenous and intracardial inoculation of tumor cells. Cancer Res 44(9):3902–3906
Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15(3):232–239. https://doi.org/10.1016/j.ccr.2009.01.021
Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15(3):220–231. https://doi.org/10.1016/j.ccr.2009.01.027
Chen L, Xiong Y, Li J, Zheng X, Zhou Q, Turner A, Wu C, Lu B, Jiang J (2017) PD-L1 expression promotes epithelial to mesenchymal transition in human esophageal cancer. Cell Physiol Biochem 42(6):2267–2280
Hirai M, Kitahara H, Kobayashi Y, Kato K, Bou Gharios G, Nakamura H, Kawashiri S (2017) Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol 50(1):41–48
Kim S, Koh J, Kim M-Y, Kwon D, Go H, Kim YA, Jeon YK, Chung DH (2016) PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol 58:7–14. https://doi.org/10.1016/j.humpath.2016.07.007
Critelli R, Milosa F, Faillaci F, Condello R, Turola E, Marzi L, Lei B, Dituri F, Andreani S, Sighinolfi P, Manni P, Maiorana A, Caporali C, di Benedetto F, Del Buono M, De Maria N, Schepis F, Martinez-Chantar M-L, Giannelli G, Villa E (2017) Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis 8:e3017. https://doi.org/10.1038/cddis.2017.395. https://www.nature.com/articles/cddis2017395#supplementary-information
McNiel EA, Tsichlis PN (2017) Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1. Signal Transduction Targeted Ther 2:16045. https://doi.org/10.1038/sigtrans.2016.45
Yao J, Caballero O, Huang Y, Lin C, Rimoldi D, Behren A, Cebon J, Hung M-C, Weinstein J, Strausberg R, Zhao Q (2016) Altered expression and splicing of ESRP1 in malignant melanoma correlates with epithelial-mesenchymal status and tumor-associated immune cytolytic activity. Cancer Immunol Res 4(6):552–561
Lou Y, Diao L, Cuentas ERP, Denning W, Chen L, Fan Y, Byers L, Wang J, Papadimitrakopoulou V, Behrens C, Rodriguez J, Hwu P, Wistuba I, Heymach J, Gibbons D (2016) Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res 22(14):3630–3642
Lou Y, Diao L, Cuentas ERP, Denning WL, Chen L, Fan Y, Byers LA, Wang J, Papadimitrakopoulou V, Behrens C, Rodriguez JC, Hwu P, Wistuba II, Heymach JV, Gibbons DL (2016) Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clinical Cancer Res 22(14):3630–3642. https://doi.org/10.1158/1078-0432.CCR-15-1434
Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, Takemoto T, Mitsudomi T (2016) Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer 98:69–75. https://doi.org/10.1016/j.lungcan.2016.04.021
Qiu X, Hu D, Chen W-Q, Chen R, Qian S, Li C, Li Y, Xiong X, Liu D, Pan F, Yu S, Chen X (2018) PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta 1864(5):1754–1769
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX-F (2014) Metastasis is regulated via microRNA-200/ZEB1 axis control of tumor cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 5:5241–5241. https://doi.org/10.1038/ncomms6241
Leduc C, Adam J, Louvet E, Sourisseau T, Dorvault N, Bernard M, Maingot E, Faivre L, Cassin Kuo M-S, Boissier E, Dessoliers M-C, Robin A, Casiraghi O, Even C, Temam S, Olaussen K, Soria J-C, Postel Vinay S (2018) TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open 3(1):e000257
Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, Semenza GL (2018) Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci USA 115(6):E1239–E1248. https://doi.org/10.1073/pnas.1718197115
Funaki S, Shintani Y, Kawamura T, Kanzaki R, Minami M, Okumura M (2017) Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep 38(4):2277–2284
Wangpaichitr M, Kandemir H, Li YY, Wu C, Nguyen DJM, Feun LG, Kuo MT, Savaraj N (2017) Relationship of metabolic alterations and PD-L1 expression in cisplatin resistant lung cancer. Cell Dev Biol 6(2):183
Jure-Kunkel M, Masters G, Girit E, Dito G, Lee F, Hunt JT, Humphrey R (2013) Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol Immunother 62(9):1533–1545. https://doi.org/10.1007/s00262-013-1451-5
Eisenhauer EA, Trudeau M (1995) An overview of phase II studies of docetaxel in patients with metastatic breast cancer. Eur J Cancer 31A(Suppl 4):S11–S13
Blum JL, Barrios CH, Feldman N, Verma S, McKenna EF, Lee LF, Scotto N, Gralow J (2012) Pooled analysis of individual patient data from capecitabine monotherapy clinical trials in locally advanced or metastatic breast cancer. Breast Cancer Res Treat 136(3):777–788. https://doi.org/10.1007/s10549-012-2288-x
Kwok W, Ling M-T, Lee T-W, Lau TCM, Zhou C, Zhang X, Chua C, Chan K, Chan F, Glackin C, Wong Y-C, Wang X (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65(12):5153–5162
Shiota M, Izumi H, Tanimoto A, Takahashi M, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Masubuchi D, Fukunaka Y, Yasuniwa Y, Naito S, Nishizawa S, Sasaguri Y, Kohno K (2009) Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res 69(7):3148–3156
van Soest RJ, de Wit R (2015) Irrefutable evidence for the use of docetaxel in newly diagnosed metastatic prostate cancer: results from the STAMPEDE and CHAARTED trials. BMC Med 13:304. https://doi.org/10.1186/s12916-015-0543-9
Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, Uesugi M, Agoulnik S, Taylor N, Funahashi Y, Matsui J (2014) Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial–mesenchymal transition (EMT) to mesenchymal–epithelial transition (MET) states. Br J Cancer 110(6):1497–1505. https://doi.org/10.1038/bjc.2014.80
Chiu LY, Hsin IL, Yang TY, Sung WW, Chi JY, Chang JT, Ko JL, Sheu GT (2017) The ERK-ZEB1 pathway mediates epithelial-mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene 36(2):242–253
Chiu LY, Hsin IL, Yang TY, Sung WW, Chi JY, Chang JT, Ko JL, Sheu GT (2017) The ERK–ZEB1 pathway mediates epithelial–mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene 36(2):242–253. https://doi.org/10.1038/onc.2016.195
Duran GE, Wang YC, Moisan F, Francisco EB, Sikic BI (2017) Decreased levels of baseline and drug-induced tubulin polymerisation are hallmarks of resistance to taxanes in ovarian cancer cells and are associated with epithelial-to-mesenchymal transition. Br J Cancer 116(10):1318–1328. https://doi.org/10.1038/bjc.2017.102
Behbahani G, Ghahhari N, Javidi M, Molan A, Feizi N, Babashah S (2017) MicroRNA-mediated post-transcriptional regulation of epithelial to mesenchymal transition in cancer. Pathol Oncol Res 23(1):1–12
Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z (2012) Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol 181(6):2188–2201. https://doi.org/10.1016/j.ajpath.2012.08.011
Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker Radtke A, McConkey D, Bar Eli M, Dinney C (2009) miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res 15(16):5060–5072
Slaby O, Laga R, Sedlacek O (2017) Therapeutic targeting of non-coding RNAs in cancer. Biochem J 474(24):4219–4251
van Zandwijk N, Pavlakis N, Kao S, Linton A, Boyer M, Clarke S, Huynh Y, Chrzanowska A, Fulham M, Bailey D, Cooper W, Kritharides L, Ridley L, Pattison S, MacDiarmid J, Brahmbhatt H, Reid G (2017) Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol 18(10):1386–1396
Zhao J, Dong D, Sun L, Zhang G (2014) Prognostic significance of the epithelial-to-mesenchymal transition markers e-cadherin, vimentin and twist in bladder cancer. Int Braz J Urol 40(2):179–189
Scherbakov AM, Andreeva OE, Shatskaya VA, Krasil’nikov MA (2012) The relationships between snail1 and estrogen receptor signaling in breast cancer cells. J Cell Biochem 113(6):2147–2155. https://doi.org/10.1002/jcb.24087
Witta S, Gemmill R, Hirsch F, Coldren C, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan D, Sugita M, Chan Z, Baron A, Franklin W, Drabkin H, Girard L, Gazdar A, Minna J, Bunn P (2006) Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res 66(2):944–950
Ren J, Chen Y, Song H, Chen L, Wang R (2013) Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J Cell Biochem 114(6):1395–1403
Garcia Bloj B, Moses C, Sgro A, Plani Lam J, Arooj M, Duffy C, Thiruvengadam S, Sorolla A, Rashwan R, Mancera R, Leisewitz A, Swift Scanlan T, Corvalan A, Blancafort P (2016) Waking up dormant tumor suppressor genes with zinc fingers, TALEs and the CRISPR/dCas9 system. Oncotarget 7(37):60535–60554
Loh YN, Hedditch EL, Baker LA, Jary E, Ward RL, Ford CE (2013) The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer 13:174. https://doi.org/10.1186/1471-2407-13-174
Buonato J, Lazzara M (2014) ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res 74(1):309–319
Gupta P, Onder T, Jiang G, Tao K, Kuperwasser C, Weinberg R, Lander E (2009) Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138(4):645–659
Qu C, Zhang W, Zheng G, Zhang Z, Yin J, He Z (2014) Metformin reverses multidrug resistance and epithelial-mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol Cell Biochem 386(1–2):63–71. https://doi.org/10.1007/s11010-013-1845-x
Saini NYX (2017) Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin 2017:1–11
Kast RE, Skuli N, Cos S, Karpel-Massler G, Shiozawa Y, Goshen R, Halatsch M-E (2017) The ABC7 regimen: a new approach to metastatic breast cancer using seven common drugs to inhibit epithelial-to-mesenchymal transition and augment capecitabine efficacy. Breast Cancer 9:495–514. https://doi.org/10.2147/BCTT.S139963
Kast R, Skuli N, Karpel Massler G, Frosina G, Ryken T, Halatsch M-E (2017) Blocking epithelial-to-mesenchymal transition in glioblastoma with a sextet of repurposed drugs: the EIS regimen. Oncotarget 8(37):60727–60749
el Ghouzzi V, Le Merrer M, Perrin Schmitt F, Lajeunie E, Benit P, Renier D, Bourgeois P, Bolcato Bellemin AL, Munnich A, Bonaventure J (1997) Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet 15(1):42–46
Chen ZF, Behringer RR (1995) twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev 9(6):686–699
Tan J, Tedrow JR, Nouraie M, Dutta JA, Miller DT, Li X, Yu S, Chu Y, Juan-Guardela B, Kaminski N, Ramani K, Biswas PS, Zhang Y, Kass DJ (2017) Loss of Twist1 in the mesenchymal compartment promotes increased fibrosis in experimental lung injury by enhanced expression of C-X-C motif ligand 12 (CXCL12). J Immunol 198(6):2269–2285. https://doi.org/10.4049/jimmunol.1600610
Palumbo ZK, Soare A, Zerr P, Liebl A, Mancuso R, Tomcik M, Sumova B, Dees C, Chen C-W, Wohlfahrt T, Mallano T, Distler A, Ramming A, Gelse K, Mihai C, Distler O, Schett G, Distler JHW (2017) Composition of TWIST1 dimers regulates fibroblast activation and tissue fibrosis. Ann Rheum Dis 76(1):244–251
Mammoto T, Jiang E, Jiang A, Lu Y, Juan AM, Chen J, Mammoto A (2013) Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering tie2 expression. PLoS ONE 8(9):e73407. https://doi.org/10.1371/journal.pone.0073407
Dong C-Y, Liu X-Y, Wang N, Wang L-N, Yang B-X, Ren Q, Liang H-Y, Ma X-T (2014) Twist-1, a novel regulator of hematopoietic stem cell self-renewal and myeloid lineage development. Stem Cells 32(12):3173–3182
Xu Y, Xu Y, Liao L, Zhou N, Theissen SM, Liao X-H, Nguyen H, Ludwig T, Qin L, Martinez JD, Jiang J, Xu J (2013) Inducible knockout of twist1 in young and adult mice prolongs hair growth cycle and has mild effects on general health, supporting twist1 as a preferential cancer target. Am J Pathol 183(4):1281–1292. https://doi.org/10.1016/j.ajpath.2013.06.021
Tukel T, Šošić D, Al-Gazali LI, Erazo M, Casasnovas J, Franco HL, Richardson JA, Olson EN, Cadilla CL, Desnick RJ (2010) Homozygous nonsense mutations in TWIST2 cause setleis syndrome. Am J Hum Genet 87(2):289–296. https://doi.org/10.1016/j.ajhg.2010.07.009
Šošić D, Richardson J, Yu K, Ornitz D, Olson E (2003) Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell 112(2):169–180
Sharabi AB, Aldrich M, Sosic D, Olson EN, Friedman AD, Lee S-H, Chen S-Y (2008) Twist-2 controls myeloid lineage development and function. PLoS Biol 6(12):e316. https://doi.org/10.1371/journal.pbio.0060316
Carver EA, Jiang R, Lan Y, Oram KF, Gridley T (2001) The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol 21(23):8184–8188. https://doi.org/10.1128/MCB.21.23.8184-8188.2001
Simon Tillaux N, Hertig A (2017) Snail and kidney fibrosis. Nephrol Dial Transplant 32(2):224–233
Shirley SH, Hudson LG, He J, Kusewitt DF (2010) The skinny on slug. Mol Carcinog 49(10):851–861. https://doi.org/10.1002/mc.20674
Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF (2009) Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J Dermatol Sci 56(1):19–26. https://doi.org/10.1016/j.jdermsci.2009.06.009
Takagi T, Moribe H, Kondoh H, Higashi Y (1998) DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 125(1):21–31
Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y (2003) Mice lacking Zfhx1b, the gene that codes for smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of hirschsprung disease–mental retardation syndrome. Am J Hum Genet 72(2):465–470
Sintov E, Nathan G, Knoller S, Pasmanik-Chor M, Russ HA, Efrat S (2015) Inhibition of ZEB1 expression induces redifferentiation of adult human β cells expanded in vitro. Sci Rep 5:13024. https://doi.org/10.1038/srep13024
Siles L, Sánchez-Tilló E, Lim J-W, Darling DS, Kroll KL, Postigo A (2013) ZEB1 imposes a temporary stage-dependent inhibition of muscle gene expression and differentiation via CtBP-mediated transcriptional repression. Mol Cell Biol 33(7):1368–1382. https://doi.org/10.1128/MCB.01259-12
Li J, Riedt T, Goossens S, Carrillo García C, Szczepanski S, Brandes M, Pieters T, Dobrosch L, Gütgemann I, Farla N, Radaelli E, Hulpiau P, Mallela N, Fröhlich H, La Starza R, Matteucci C, Chen T, Brossart P, Mecucci C, Huylebroeck D, Haigh J, Janzen V (2017) The EMT transcription factor Zeb2 controls adult murine hematopoietic differentiation by regulating cytokine signaling. Blood 129(4):460–472
Weng Q, Chen Y, Wang H, Xu X, Yang B, He Q, Shou W, Chen Y, Higashi Y, van den Berghe V, Seuntjens E, Kernie SG, Bukshpun P, Sherr EH, Huylebroeck D, Lu QR (2012) Dual-mode modulation of smad signaling by smad-interacting protein Sip1 is required for myelination in the CNS. Neuron 73(4):713–728. https://doi.org/10.1016/j.neuron.2011.12.021
Denecker G, Vandamme N, Akay Ö, Koludrovic D, Taminau J, Lemeire K, Gheldof A, De Craene B, Van Gele M, Brochez L, Udupi GM, Rafferty M, Balint B, Gallagher WM, Ghanem G, Huylebroeck D, Haigh J, van den Oord J, Larue L, Davidson I, Marine JC, Berx G (2014) Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ 21(8):1250–1261. https://doi.org/10.1038/cdd.2014.44
Haydon RC, Luu HH, He T-C (2007) Osteosarcoma and osteoblastic differentiation: a new perspective on oncogenesis. Clin Orthop Relat Res 454:237–246. https://doi.org/10.1097/BLO.0b013e31802b683c
Ishikawa T, Shimizu T, Ueki A, Yamaguchi S, Onishi N, Sugihara E, Kuninaka S, Miyamoto T, Morioka H, Nakayama R, Kobayashi E, Toyama Y, Mabuchi Y, Matsuzaki Y, Yamaguchi R, Miyano S, Saya H (2013) Twist2 functions as a tumor suppressor in murine osteosarcoma cells. Cancer Sci 104(7):880–888
Man T-K, Chintagumpala M, Visvanathan J, Shen J, Perlaky L, Hicks J, Johnson M, Davino N, Murray J, Helman L, Meyer W, Triche T, Wong K-K, Lau CC (2005) Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer Res 65(18):8142–8150. https://doi.org/10.1158/0008-5472.Can-05-0985
Yang H, Zhang Y, Zhou Z, Jiang X, Shen A (2011) Snail-1 regulates VDR signaling and inhibits 1,25(OH)-D3 action in osteosarcoma. Eur J Pharmacol 670(2):341–346. https://doi.org/10.1016/j.ejphar.2011.09.160
Larriba MJ, Bonilla F, Muñoz A (2010) The transcription factors Snail1 and Snail2 repress vitamin D receptor during colon cancer progression. J Steroid Biochem Mol Biol 121(1):106–109. https://doi.org/10.1016/j.jsbmb.2010.01.014
Sharili A-S, Allen S, Smith K, Hargreaves J, Price J, McGonnell I (2011) Expression of Snail2 in long bone osteosarcomas correlates with tumour malignancy. Tumor Biol 32(3):515–526. https://doi.org/10.1007/s13277-010-0146-1
Aidong S, Yunqing Z, Huiguang Y, Ruisheng X, Guowei H (2012) Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J Surg Oncol 105(8):830–834. https://doi.org/10.1002/jso.23012
Wensman H, Göransson H, Leuchowius K-J, Strömberg S, Pontén F, Isaksson A, Rutteman GR, Heldin N-E, Pejler G, Hellmén E (2009) Extensive expression of craniofacial related homeobox genes in canine mammary sarcomas. Breast Cancer Res Treat 118(2):333–343. https://doi.org/10.1007/s10549-008-0243-7
Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, Guerci A, Duarte M, Daniel MT, Bowen D (1993) Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91. Group. Blood 82(11):3241–3249
Dutcher J, Lee S, Gallagher R, Makary A, Hines J, Londer H, Farnen J, Bennett J, Paietta E, Rowe J, Goloubeva O, Wiernik P (2005) Phase II study of all-trans retinoic acid in the accelerated phase or early blastic phase of chronic myeloid leukemia: a study of the Eastern Cooperative Oncology Group (E1993). Leuk Lymphoma 46(3):377–385
Visani G, Tosi P, Manfroi S, Ottaviani E, Finelli C, Cenacchi A, Bendandi M, Tura S (1995) All-trans retinoic acid in the treatment of myelodysplastic syndromes. Leuk Lymphoma 19(3–4):277–280
Yang J-Z, Lian W-G, Sun L-X, Qi D-W, Ding Y, Zhang X-H (2016) High nuclear expression of Twist1 in the skeletal extramedullary disease of myeloma patients predicts inferior survival. Pathol Res Pract 212(3):210–216
Cosset E, Hamdan G, Jeanpierre S, Voeltzel T, Sagorny K, Hayette S, Mahon F-X, Dumontet C, Puisieux A, Nicolini F, Maguer Satta V (2011) Deregulation of TWIST-1 in the CD34 + compartment represents a novel prognostic factor in chronic myeloid leukemia. Blood 117(5):1673–1676
Xin H, Kong Y, Jiang X, Wang K, Qin X, Miao Z-H, Zhu Y, Tan W (2013) Multi-drug-resistant cells enriched from chronic myeloid leukemia cells by Doxorubicin possess tumor-initiating-cell properties. J Pharmacol Sci 122(4):299–304
Mancini M, Petta S, Iacobucci I, Salvestrini V, Barbieri E, Santucci M (2010) Zinc-finger transcription factor slug contributes to the survival advantage of chronic myeloid leukemia cells. Cell Signal 22(8):1247–1253
Sun Y, Pan J, Mao S, Jin J (2014) IL-17/miR-192/IL-17Rs regulatory feedback loop facilitates multiple myeloma progression. PLoS ONE 9(12):e114647
Lemma S, Karihtala P, Haapasaari K-M, Jantunen E, Soini Y, Bloigu R, Pasanen A-K, Turpeenniemi Hujanen T, Kuittinen O (2013) Biological roles and prognostic values of the epithelial-mesenchymal transition-mediating transcription factors Twist, ZEB1 and Slug in diffuse large B-cell lymphoma. Histopathology 62(2):326–333
Stavropoulou V, Kaspar S, Brault L, Sanders M, Juge S, Morettini S, Tzankov A, Iacovino M, Lau IJ, Milne T, Royo H, Kyba M, Valk PJM, Peters AHFM., Schwaller J (2016) MLL-AF9 expression in hematopoietic stem cells drives a highly invasive AML expressing emt-related genes linked to poor outcome. Cancer Cell 30(1):43–58
Zhang X, Ma W, Cui J, Yao H, Zhou H, Ge Y, Xiao L, Hu X, Liu BH, Yang J, Li YY, Chen S, Eaves CJ, Wu D, Zhao Y (2015) Regulation of p21 by TWIST2 contributes to its tumor-suppressor function in human acute myeloid leukemia. Oncogene 34(23):3000–3010
Thathia S, Ferguson S, Gautrey H, van Otterdijk S, Hili M, Rand V, Moorman A, Meyer S, Brown R, Strathdee G (2012) Epigenetic inactivation of TWIST2 in acute lymphoblastic leukemia modulates proliferation, cell survival and chemosensitivity. Haematologica 97(3):371–378
Nakahata S, Yamazaki S, Nakauchi H, Morishita K (2010) Downregulation of ZEB1 and overexpression of Smad7 contribute to resistance to TGF-beta1-mediated growth suppression in adult T-cell leukemia/lymphoma. Oncogene 29(29):4157–4169
Mishra A, La Perle K, Kwiatkowski S, Sullivan L, Sams G, Johns J, Curphey D, Wen J, McConnell K, Qi J, Wong H, Russo G, Zhang J, Marcucci G, Bradner J, Porcu P, Caligiuri M (2016) Mechanism, consequences, and therapeutic targeting of abnormal IL15 signaling in cutaneous T-cell lymphoma. Cancer Discov 6(9):986–1005
Cui J, Gong M, He Y, Li Q, He T, Bi Y (2016) All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1-6 cells through reversing EMT in vitro. Int J Oncol 48(1):349–357
Zanetti A, Affatato R, Centritto F, Fratelli M, Kurosaki M, Barzago MM, Bolis M, Terao M, Garattini E, Paroni G (2015) All-trans-retinoic acid modulates the plasticity and inhibits the motility of breast cancer cells: role of Notch1 and transforming growth factor (TGFβ). J Biol Chem 290(29):17690–17709. https://doi.org/10.1074/jbc.M115.638510
Lan L, Deng W, Chen HL, Huo LL, Deng LL, Zhang GY, Luo Y (2016) All-trans retinoic acid improves iodine uptake of thyroid cancer cells via repressing transcriptional activity of β-catenin. Zhonghua yi Xue za Zhi 96(7):553–558
Schultze E, Collares T, Lucas CG, Seixas FK (2018) Synergistic and additive effects of ATRA in combination with different anti-tumor compounds. Chem Biol Interact 285:69–75. https://doi.org/10.1016/j.cbi.2018.02.021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Redfern, A.D., Spalding, L.J. & Thompson, E.W. The Kraken Wakes: induced EMT as a driver of tumour aggression and poor outcome. Clin Exp Metastasis 35, 285–308 (2018). https://doi.org/10.1007/s10585-018-9906-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-018-9906-x