Abstract
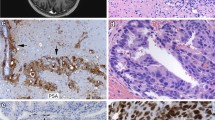
TGFβ is a known driver of epithelial-mesenchymal transition (EMT) which is associated with tumor aggressiveness and metastasis. However, EMT has not been fully explored in clinical specimens of castration-resistant prostate cancer (CRPC) metastases. To assess EMT in CRPC, gene expression analysis was performed on 149 visceral and bone metastases from 62 CRPC patients and immunohistochemical analysis was performed on 185 CRPC bone and visceral metastases from 42 CRPC patients. In addition, to assess the potential of metastases to seed further metastases the mitochondrial genome was sequenced at different metastatic sites in one patient. TGFβ was increased in bone versus visceral metastases. While primarily cytoplasmic; nuclear and cytoplasmic Twist were significantly higher in bone than in visceral metastases. Slug and Zeb1 were unchanged, with the exception of nuclear Zeb1 being significantly higher in visceral metastases. Importantly, nuclear Twist, Slug, and Zeb1 were only present in a subset of epithelial cells that had an EMT-like phenotype. Underscoring the relevance of EMT-like cells, mitochondrial sequencing revealed that metastases could seed additional metastases in the same patient. In conclusion, while TGFβ expression and EMT-associated protein expression is present in a considerable number of CRPC visceral and bone metastases, nuclear Twist, Slug, and Zeb1 localization and an EMT-like phenotype (elongated nuclei and cytoplasmic compartment) was only present in a small subset of CRPC bone metastases. Mitochondrial sequencing from different metastases in a CRPC patient provided evidence for the seeding of metastases from previously established metastases, highlighting the biological relevance of EMT-like behavior in CRPC metastases.




Similar content being viewed by others
References
Anose BM, LaGoo L, Schwendinger J (2008) Characterization of androgen regulation of ZEB-1 and PSA in 22RV1 prostate cancer cells. Adv Exp Med Biol 617:541–546
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, De Garcia HA (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89
Behnsawy HM, Miyake H, Harada K, Fujisawa M (2013) Expression patterns of epithelial-mesenchymal transition markers in localized prostate cancer: significance in clinicopathological outcomes following radical prostatectomy. BJU Int 111:30–37
Boyer B, Tucker GC, Valles AM, Franke WW, Thiery JP (1989) Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol 109:1495–1509
Bryden AA, Hoyland JA, Freemont AJ, Clarke NW, Schembri WD, George NJ (2002) E-cadherin and beta-catenin are down-regulated in prostatic bone metastases. BJU Int 89:400–403
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:179
Das S, Becker BN, Hoffmann FM, Mertz JE (2012) Reversal of transforming growth factor-beta induced epithelial-to-mesenchymal transition and the ZEB proteins. Fibrogenesis Tissue Repair 5:S28
De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI (1999) E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology 53:707–713
Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29:117–129
Drake JM, Barnes JM, Madsen JM, Domann FE, Stipp CS, Henry MD (2010) ZEB1 coordinately regulates laminin-332 and {beta}4 integrin expression altering the invasive phenotype of prostate cancer cells. J Biol Chem 285:33940–33948
Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD (2009) ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell 20:2207–2217
Dumont N, Arteaga CL (2003) Targeting the TGF beta signaling network in human neoplasia. Cancer Cell 3:531–536
Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R (2005) DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24:2375–2385
Emadi BM, Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W, Schulz WA (2010) Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol 31:297–307
Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K (1982) Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation 23:43–59
Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566:9–20
Hugo HJ, Kokkinos MI, Blick T, Ackland ML, Thompson EW, Newgreen DF (2011) Defining the E-cadherin repressor interactome in epithelial-mesenchymal transition: the PMC42 model as a case study. Cells Tissues Organs 193:23–40
Keshamouni VG, Schiemann WP (2009) Epithelial-mesenchymal transition in tumor metastasis: a method to the madness. Future Oncol 5:1109–1111
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH (2009) miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 27:1712–1721
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, Wong YC, Wang X (2005) Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res 65:5153–5162
Larson SR, Zhang X, Dumpit R, Coleman I, Lakely B, Roudier M, Higano CS, True LD, Lange PH, Montgomery B, Corey E, Nelson PS, Vessella RL, Morrissey C (2013) Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate 73:932–940
Liu GL, Yang HJ, Liu T, Lin YZ (2014) Expression and significance of E-cadherin, N-cadherin, transforming growth factor-beta1 and Twist in prostate cancer. Asian Pac J Trop Med 7:76–82
Luo Y, He DL, Ning L (2006) Expression of “epithelial-mesenchymal transition” associated proteins in prostate cancer cell lines with different metastatic potentials and its significance. Zhonghua Nan Ke Xue 12:696–700
Morrissey C, Roudier MP, Dowell A, True LD, Ketchanji M, Welty C, Corey E, Lange PH, Higano CS, Vessella RL (2013) Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: results from the University of Washington Rapid Autopsy Series. J Bone Miner Res 28:333–340
Nauseef JT, Henry MD (2011) Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nat Rev Urol 8:428–439
Pontes J Jr, Srougi M, Borra PM, Dall’ Oglio MF, Ribeiro-Filho LA, Leite KR (2010) E-cadherin and beta-catenin loss of expression related to bone metastasis in prostate cancer. Appl Immunohistochem Mol Morphol 18:179–184
Putzke AP, Ventura AP, Bailey AM, Akture C, Opoku-Ansah J, Celiktas M, Hwang MS, Darling DS, Coleman IM, Nelson PS, Nguyen HM, Corey E, Tewari M, Morrissey C, Vessella RL, Knudsen BS (2011) Metastatic progression of prostate cancer and e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol 179:400–410
Qiao B, Johnson NW, Gao J (2010) Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J Oncol 37:663–668
Raatikainen S, Aaltomaa S, Palvimo JJ, Karja V, Soini Y (2014) TWIST overexpression predicts biochemical recurrence-free survival in prostate cancer patients treated with radical prostatectomy. Scand J Urol 49:51
Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL (2003) Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol 34:646–653
Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML (2001) E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol 32:690–697
Salas A, Yao YG, Macaulay V, Vega A, Carracedo A, Bandelt HJ (2005) A critical reassessment of the role of mitochondria in tumorigenesis. PLoS Med 2:e296
Slabakova E, Pernicova Z, Slavickova E, Starsichova A, Kozubik A, Soucek K (2011) TGF-beta1-induced EMT of non-transformed prostate hyperplasia cells is characterized by early induction of SNAI2/Slug. Prostate 71:1332–1343
Subramanian G, Schwarz RE, Higgins L, McEnroe G, Chakravarty S, Dugar S, Reiss M (2004) Targeting endogenous transforming growth factor beta receptor signaling in SMAD4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype1. Cancer Res 64:5200–5211
Tarin D, Thompson EW, Newgreen DF (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65:5996–6000
Taylor RW, Taylor GA, Durham SE, Turnbull DM (2001) The determination of complete human mitochondrial DNA sequences in single cells: implications for the study of somatic mitochondrial DNA point mutations. Nucleic Acids Res 29:E74
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98:5116–5121
Vandewalle C, Comijn J, De CB, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van RF, Berx G (2005) SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res 33:6566–6578
Wallerand H, Robert G, Pasticier G, Ravaud A, Ballanger P, Reiter RE, Ferriere JM (2010) The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urol Oncol 28:473–479
Wu KJ, Zeng J, Zhu GD, Zhang D, Xue Y, Chen YL, Wang XY, He DL (2010) Comparison of transcription factors repressing epithelial phenotype in two different prostate cancer EMT models and its significance. Zhonghua Nan Ke Xue 16:137–141
Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, Chung LW, Zhau HE (2006) Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 66:1664–1673
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939
Yingling JM, Blanchard KL, Sawyer JS (2004) Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov 3:1011–1022
Yu J, Xie F, Bao X, Chen W, Xu Q (2014) miR-300 inhibits epithelial to mesenchymal transition and metastasis by targeting Twist in human epithelial cancer. Mol Cancer 13:121
Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW (2007) Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology 50:648–658
Zavadil J, Bottinger EP (2005) TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24:5764–5774
Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP (2004) Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23:1155–1165
Zhang X, Morrissey C, Sun S, Ketchandji M, Nelson PS, True LD, Vakar-Lopez F, Vessella RL, Plymate SR (2011) Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE 6:e27970
Zhu ML, Kyprianou N (2010) Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 24:769–777
Acknowledgments
We would like to thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program, for without them research of this nature would not be possible. Additionally, we would also like to thank Khanhthy Doan, Funda Vakar-Lopez, Maria Tretiakova, Evan Yu, Elahe Mostaghel and the rapid autopsy teams in the Urology Department at the University of Washington. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington (RLV is a VA Biomedical Laboratory R&D Senior Research Career Scientist, PHL is a Staff Physician), the Pacific Northwest Prostate Cancer SPORE (P50CA97186), the PO1 NIH grant (PO1CA085859), the LUCAS Foundation, W81XWH-10-1-0563 from the CDMRP/U.S. Department of Defense, and an Outstanding New Environmental Scientist Award (ONES) (R01) from the National Institute of Environmental Health Sciences (R01 ES019319). CM is a recipient of a Career Development Award from Jim and Cathrine Allchin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2015_9773_MOESM1_ESM.tif
Supplementary material 1 (TIFF 16253 kb) Representative IHC images of TGFβ and Pan-cytokeratin expression in 5 different bone metastases. White arrows highlight epithelial cells with elongated nuclei and the black arrows highlight cells with round nuclei. Bar = 40 microns
10585_2015_9773_MOESM2_ESM.tif
Supplementary material 2 (TIFF 16459 kb) Representative IHC images of Twist and Pan-cytokeratin expression in 5 different bone metastases. White arrows highlight epithelial cells with elongated nuclei and the black arrows highlight cells with round nuclei. Bar = 40 microns
10585_2015_9773_MOESM3_ESM.tif
Supplementary material 3 (TIFF 16695 kb) Representative IHC images of Slug and Pan-cytokeratin expression in 5 different bone metastases. White arrows highlight epithelial cells with elongated nuclei and the black arrows highlight cells with round nuclei. Bar = 40 microns
10585_2015_9773_MOESM4_ESM.tif
Supplementary material 4 (TIFF 16509 kb) Representative IHC images of Zeb1 and Pan-cytokeratin expression in 5 different bone metastases. White arrows highlight epithelial cells with elongated nuclei and the black arrows highlight cells with round nuclei. Bar = 40 microns
10585_2015_9773_MOESM5_ESM.tif
Supplementary material 5 (TIFF 16472 kb) Vimentin was not expressed in the cytoplasm or nuclei of epithelial cancer cells, but did stain in the stromal elements. White arrows highlight epithelial cells with elongated nuclei and the black arrows highlight cells with round nuclei. Bar = 40 microns
10585_2015_9773_MOESM6_ESM.tif
Supplementary material 6 (TIFF 5377 kb) A schematic representation of the mitochondrial genome in metastases from a patient with CRPC. The mitochondrial genome from two normal tissues, the primary tumor, and fifteen metastatic sites was sequenced from one patient. Five sites had a mutation in ND1, eight sites had a mutation in ND1 and ND5, with two further sites having an additional mutation in a tRNA and ND2 respectively
10585_2015_9773_MOESM7_ESM.tif
Supplementary material 7 (TIFF 27464 kb) Representative IHC images of the androgen receptor and Pan-cytokeratin expression in 5 different bone metastases. Black arrows highlight epithelial cells with elongated nuclei. Bar = 20 microns
Rights and permissions
About this article
Cite this article
Haider, M., Zhang, X., Coleman, I. et al. Epithelial mesenchymal-like transition occurs in a subset of cells in castration resistant prostate cancer bone metastases. Clin Exp Metastasis 33, 239–248 (2016). https://doi.org/10.1007/s10585-015-9773-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9773-7