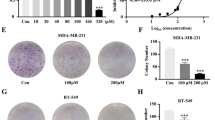
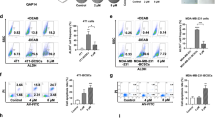
Abstract
Dipyridamole is a widely prescribed drug in ischemic disorders, and it is here investigated for potential clinical use as a new treatment for breast cancer. Xenograft mice bearing triple-negative breast cancer 4T1-Luc or MDA-MB-231T cells were generated. In these in vivo models, dipyridamole effects were investigated for primary tumor growth, metastasis formation, cell cycle, apoptosis, signaling pathways, immune cell infiltration, and serum inflammatory cytokines levels. Dipyridamole significantly reduced primary tumor growth and metastasis formation by intraperitoneal administration. Treatment with 15 mg/kg/day dipyridamole reduced mean primary tumor size by 67.5 % (p = 0.0433), while treatment with 30 mg/kg/day dipyridamole resulted in an almost a total reduction in primary tumors (p = 0.0182). Experimental metastasis assays show dipyridamole reduces metastasis formation by 47.5 % in the MDA-MB-231T xenograft model (p = 0.0122), and by 50.26 % in the 4T1-Luc xenograft model (p = 0.0292). In vivo dipyridamole decreased activated β-catenin by 38.64 % (p < 0.0001), phospho-ERK1/2 by 25.05 % (p = 0.0129), phospho-p65 by 67.82 % (p < 0.0001) and doubled the expression of IkBα (p = 0.0019), thus revealing significant effects on Wnt, ERK1/2-MAPK and NF-kB pathways in both animal models. Moreover dipyridamole significantly decreased the infiltration of tumor-associated macrophages and myeloid-derived suppressor cells in primary tumors (p < 0.005), and the inflammatory cytokines levels in the sera of the treated mice. We suggest that when used at appropriate doses and with the correct mode of administration, dipyridamole is a promising agent for breast-cancer treatment, thus also implying its potential use in other cancers that show those highly activated pathways.









Similar content being viewed by others
Abbreviations
- AGP:
-
α1 Acid glycoprotein
- BCRP/ABCG2:
-
Human breast cancer resistance protein
- BLI:
-
Bioluminescence imaging
- CI:
-
Cell index
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethylsulfoxide
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- IHC:
-
Immunohistochemistry
- IL-1α:
-
Interleukin-1α
- IL-1β:
-
Interleukin-1β
- MCP-1:
-
Monocyte chemotactic protein 1
- MDSCs:
-
Myeloid-derived suppressor cells
- MIP-1a:
-
Macrophage inflammatory protein 1a
- MMP9:
-
Matrix metalloproteinase 9
- MTS:
-
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- PBS:
-
Phosphate-buffered saline
- PEG:
-
Polyethylene glycol
- PGK:
-
Phosphoglucokinase
- RTCA:
-
Real-time cell analysis
- RT-CES:
-
Real-time cell electronic sensor
- SCF:
-
Stem cell factor
- SE:
-
Standard error
- SEM:
-
Standard error of the mean
- TAMs:
-
Tumor-associated macrophages
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
Schaper W (2005) Dipyridamole, an underestimated vascular protective drug. Cardiovasc Drugs Ther 19:357–363
Belt JA, Marina NM, Phelps DA, Crawford CR (1993) Nucleoside transport in normal and neoplastic cells. Adv Enzyme Regul 33:235–252
Walling J (2006) From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Invest New Drugs 24:37–77
Howell SB, Hom D, Sanga R, Vick JS, Abramson IS (1989) Comparison of the synergistic potentiation of etoposide, doxorubicin, and vinblastine cytotoxicity by dipyridamole. Cancer Res 49:3178–3183
Ramu N, Ramu A (1989) Circumvention of adriamycin resistance by dipyridamole analogues: a structure-activity relationship study. Int J Cancer 43:487–491
Lehman NL, Danenberg PV (2000) Modulation of RTX cytotoxicity by thymidine and dipyridamole in vitro: implications for chemotherapy. Cancer Chemother Pharmacol 45:142–148
Nelson JA, Drake S (1984) Potentiation of methotrexate toxicity by dipyridamole. Cancer Res 44:2493–2496
Desai PB, Sridhar R (1992) Potentiation of cytotoxicity of mitoxantrone toward CHO-K1 cells in vitro by dipyridamole. Pharm Res 9:178–181
Boyer CR, Karjian PL, Wahl GM, Pegram M, Neuteboom ST (2002) Nucleoside transport inhibitors, dipyridamole and p-nitrobenzylthioinosine, selectively potentiate the antitumor activity of NB1011. Anticancer Drugs 13:29–36
Rodrigues M, Barbosa F Jr, Perussi JR (2004) Dipyridamole increases the cytotoxicity of cisplatin in human larynx cancer cells in vitro. Braz J Med Biol Res 37:591–599
Sato S, Kohno K, Hidaka K, Hisatsugu T, Kuwano M, Komiyama S (1993) Differentially potentiating effects by dipyridamole on cytotoxicity of 5-fluorouracil against three human maxillary cancer cell lines derived from a single tumor. Anticancer Drug Des 8:289–297
Kennedy DG, Van den Berg HW, Clarke R, Murphy RF (1986) Enhancement of methotrexate cytotoxicity towards the MDA.MB.436 human breast cancer cell line by dipyridamole. The role of methotrexate polyglutamates. Biochem Pharmacol 35:3053–3056
Goda AE, Yoshida T, Horinaka M, Yasuda T, Shiraishi T, Wakada M, Sakai T (2008) Mechanisms of enhancement of TRAIL tumoricidal activity against human cancer cells of different origin by dipyridamole. Oncogene 27:3435–3445
Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, Mao Q (2005) BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res 22:2023–2034
Haimeur A, Conseil G, Deeley RG, Cole SP (2004) The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab 5:21–53
Eisert WG (2002) Dipyridamole. In: Michelson AD (ed) Platelets. Academic Press, Amsterdam, pp 803–815
White H, Jamieson DG (2010) Review of the ESPRIT Study: aspirin plus dipyridamole versus aspirin alone for prevention of vascular events after a noncardioembolic, mild-to-moderate ischemic stroke or transient ischemic attack. Postgrad Med 122:227–229
Tsuruo T, Fujita N (2008) Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci 84:189–198
Nierodzik ML, Karpatkin S (2006) Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 10:355–362
Isacoff WH, Bendetti JK, Barstis JJ, Jazieh AR, Macdonald JS, Philip PA (2007) Phase II trial of infusional fluorouracil, leucovorin, mitomycin, and dipyridamole in locally advanced unresectable pancreatic adenocarcinoma: SWOG S9700. J Clin Oncol 25:1665–1669
Raschko JW, Synold TW, Chow W, Coluzzi P, Hamasaki V, Leong LA, Margolin KA, Morgan RJ, Shibata SI, Somlo G, Tetef ML, Yen Y, ter Veer A, Doroshow JH (2000) A phase I study of carboplatin and etoposide administered in conjunction with dipyridamole, prochlorperazine and cyclosporine A. Cancer Chemother Pharmacol 46:403–410
Burch PA, Ghosh C, Schroeder G, Allmer C, Woodhouse CL, Goldberg RM, Addo F, Bernath AM, Tschetter LK, Windschitl HE, Cobau CD (2000) Phase II evaluation of continuous-infusion 5-fluorouracil, leucovorin, mitomycin-C, and oral dipyridamole in advanced measurable pancreatic cancer: a North Central Cancer Treatment Group Trial. Am J Clin Oncol 23:534–537
Wenzel J, Zeisig R, Fichtner I (2009) Inhibition of breast cancer metastasis by dual liposomes to disturb complex formation. Int J Pharm 370:121–128
Wenzel J, Zeisig R, Haider W, Habedank S, Fichtner I (2010) Inhibition of pulmonary metastasis in a human MT3 breast cancer xenograft model by dual liposomes preventing intravasal fibrin clot formation. Breast Cancer Res Treat 121:13–22
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948
Xing JZ, Zhu L, Gabos S, Xie L (2006) Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol in Vitro 20:995–1004
Spano D, Cimmino F, Capasso M, D’Angelo F, Zambrano N, Terracciano L, Iolascon A (2008) Changes of the hepatic proteome in hepatitis B-infected mouse model at early stages of fibrosis. J Proteome Res 7:2642–2653
Chuang KA, Lieu CH, Tsai WJ, Wu MH, Chen YC, Liao JF, Wang CC, Kuo YC (2010) Evaluation of anti-Wnt/β-catenin signaling agents by pGL4-TOP transfected stable cells with a luciferase reporter system. Braz J Med Biol Res 43:931–941
Vlad A, Röhrs S, Klein-Hitpass L, Müller O (2008) The first five years of the Wnt targetome. Cell Signal 20:795–802
Weyrich AS, Denis MM, Kuhlmann-Eyre JR, Spencer ED, Dixon DA, Marathe GK, McIntyre TM, Zimmerman GA, Prescott SM (2005) Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation 111:633–642
Chakrabarti S, Blair P, Wu C, Freedman JE (2007) Redox state of dipyridamole is a critical determinant for its beneficial antioxidant and antiinflammatory effects. J Cardiovasc Pharmacol 50:449–457
Sethi G, Ahn KS, Sung B, Aggarwal BB (2008) Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol Cancer Ther 7:1604–1614
Kawakami H, Tomita M, Matsuda T, Ohta T, Tanaka Y, Fujii M, Hatano M, Tokuhisa T, Mori N (2005) Transcriptional activation of survivin through the NF-kappaB pathway by human T-cell leukemia virus type I tax. Int J Cancer 115:967–974
Mantovani A (2010) Molecular pathways linking inflammation and cancer. Curr Mol Med 10:369–373
Boye K, Maelandsmo GM (2010) S100A4 and metastasis: a small actor playing many roles. Am J Pathol 176:528–535
Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH (2006) The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131:1486–1500
Grotterød I, Maelandsmo GM, Boye K (2010) Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-kappaB. BMC Cancer 10:241
Haskó G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabó C (2000) Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 14:2065–2074
Budd GT, Jayaraj A, Grabowski D, Adelstein D, Bauer L, Boyett J, Bukowski R, Murthy S, Weick J (1990) Phase I trial of dipyridamole with 5-fluorouracil and folinic acid. Cancer Res 50:7206–7211
Chakravarthy A, Abrams RA, Yeo CJ, Korman LT, Donehower RC, Hruban RH, Zahurek ML, Grochow LB, O’Reilly S, Hurwitz H, Jaffee EM, Lillemoe KD, Cameron JL (2000) Intensified adjuvant combined modality therapy for resected periampullary adenocarcinoma: acceptable toxicity and suggestion of improved 1-year disease-free survival. Int J Radiat Oncol Biol Phys 48:1089–1096
Willson JK, Fischer PH, Tutsch K, Alberti D, Simon K, Hamilton RD, Bruggink J, Koeller JM, Tormey DC, Earhart RH, Ranhosky A, Trump DL (1988) Phase I clinical trial of a combination of dipyridamole and acivicin based upon inhibition of nucleoside salvage. Cancer Res 48:5585–5590
Goel R, Cleary SM, Horton C, Balis FM, Zimm S, Kirmani S, Howell SB (1989) Selective intraperitoneal biochemical modulation of methotrexate by dipyridamole. J Clin Oncol 7:262–269
Isonishi S, Kirmani S, Kim S, Plaxe SC, Braly PS, McClay EF, Howell SB (1991) Phase I and pharmacokinetic trial of intraperitoneal etoposide in combination with the multidrug-resistance-modulating agent dipyridamole. J Natl Cancer Inst 83:621–626
Mahony C, Wolfram KM, Cocchetto DM, Bjornsson TD (1982) Dipyridamol kinetics. Clin Pharmacol Ther 31:330–338
Curtin NJ, Bowman KJ, Turner RN, Huang B, Loughlin PJ, Calvert AH, Golding BT, Griffin RJ, Newell DR (1999) Potentiation of the cytotoxicity of thymidylate synthase (TS) inhibitors by dipyridamole analogues with reduced alpha1-acid glycoprotein binding. Br J Cancer 80:1738–1746
Huang C, Jacobson K, Schaller MD (2004) MAP kinases and cell migration. J Cell Sci 117:4619–4628
Kaler P, Godasi BN, Augenlicht L, Klampfer L (2009) The NF-kappaB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1beta. Cancer Microenviron 2:69–80
Kim D, Rath O, Kolch W, Cho KH (2007) A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways. Oncogene 26:4571–4579
Chuderland D, Seger R (2005) Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biotechnol 29:57–74
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86:1065–1073
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461–466
Acknowledgments
We would like to thank: Prof. Eugene Lukanidin for sharing the S100A4 antibodies used in this study, Prof. Luigi del Vecchio and Dr. Maddalena Raia for technical advice with the FACS analyses, Dr. Donatella Montanaro for technical advice with histological analyses, and Prof. Francesco Salvatore for supporting the project with the instrumentation required for in vivo imaging in mice. We also thank Viviana Vastolo for technical assistance in in vivo experiments. Associazione Italiana per la ricerca sul Cancro AIRC (MZ), Associazione Italiana per la lotta al Neuroblastoma (MZ). This study was also supported in part by the Intramural Research Program of the National Cancer Institute (PSS). DS was supported by the Dipartimento di Biochimica e Biotecnologie Mediche, ‘Federico II’ University of Naples; VDD was supported by the Fondazione San Paolo (IM) and Tumic; DMD was supported by a Dottorato in Medicina Molecolare, ‘Federico II’ University of Naples; GDV was supported by a Dottorato in Medicina Molecolare, ‘Federico II’ University of Naples; and CM was supported by a Dottorato in Produzione e Sanità degli Alimenti di Origine Animale, ‘Federico II’ University of Naples.
Conflict of interest
The authors declare that they have no competing interests as defined by Clinical & Experimental Metastasis, or other interests that might be perceived as influencing the results and discussion reported in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniela De Martino, Alessia Romano and Maria Nunzia Scoppettuolo contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2012_9506_MOESM1_ESM.tif
Figure S1. Representative caspase-3 activity assay (of two experiments performed with similar results) in 4T1-Luc cells treated for 24 h with PBS-PEG or dipyridamole (as indicated). Data are means ±SE. * p = 0.03 (Students’ t-test). (TIFF 16188 kb)
10585_2012_9506_MOESM2_ESM.tif
Figure S2. Dipyridamole affects 4T1-Luc primary tumor growth in vivo. Time-course of bioluminescent signals from 4T1-Luc tumor cells implanted into the mouse mammary fat pad at day 0, followed by intraperitoneally administered treatments with PBS-PEG or 15 mg/kg/day (A) or 30 mg/kg/day (B) dipyridamole (as indicated). Data are total flux means ±SE. (A) * p = 0.0433; (B) * p = 0.0182 (ANOVA). (TIFF 16184 kb)
10585_2012_9506_MOESM3_ESM.tif
Figure S3. In vivo effects of dipyridamole. Statistical analyses on sections from 4T1-Luc primary tumors (A) and MDA-MB-231T lung metastases (B, C) stained for cleaved caspase-3 (A, C) and Ki67 (B). No significant differences were observed for all of the analyzed markers. (TIFF 16453 kb)
10585_2012_9506_MOESM4_ESM.tif
Figure S4. Dipyridamole toxicity in vivo. Time-course of mean weights of athymic nude mice injected with MDA-MB-231T cells via the tail vein and treated with vehicle or 30 mg/kg/day dipyridamole, delivery either by oral gavage or intraperitoneally (as indicated). There are no significant differences between the body weights of these three treatment groups. (TIFF 16194 kb)
10585_2012_9506_MOESM5_ESM.tif
Figure S5. In vitro effects of dipyridamole. (A) Immunoblotting for IkBα in 4T1-Luc cells treated for 4 h with PBS-PEG or dipyridamole (as indicated). β-actin used as control for equal loading. (B) Expression of PGK gene, a non-target of Wnt, ERK1/2-MAPK and NF-kB pathways, in 4T1-Luc cells treated for 24 h with PBS-PEG or dipyridamole (as indicated). Data are means ±SEM. (TIFF 16189 kb)
10585_2012_9506_MOESM6_ESM.tif
Figure S6. Dipyridamole affects in vivo the expression of known targets of Wnt pathway. Immunoblotting for cyclin D1 and c-Myc in tumors from mice implanted with 4T1-Luc cells in the mammary fat pad and treated with PBS-PEG or dipyridamole (as indicated). β-actin used as control for equal loading. (TIFF 16188 kb)
Rights and permissions
About this article
Cite this article
Spano, D., Marshall, JC., Marino, N. et al. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis 30, 47–68 (2013). https://doi.org/10.1007/s10585-012-9506-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9506-0