Abstract
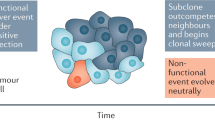
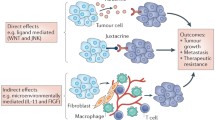
The progression of tumours to malignancy is commonly considered to arise through lineal evolution, a process in which mutations conferring pro-oncogenic cellular phenotypes are acquired by a succession of ever-more dominant clones. However, this model is at odds with the persistent polyclonality observed in many cancers. We propose that an alternative mechanism for tumour progression, called interclonal cooperativity, is likely to play a role at stages of tumour progression when mutations cause microenvironmental changes, such as occur with epithelial-mesenchymal transitions (EMTs). Interclonal cooperativity occurs when cancer cell–cancer cell interactions produce an emergent malignant phenotype from individually non-malignant clones. In interclonal cooperativity, the oncogenic mutations occur in different clones within the tumour that complement each other and cooperate in order to drive progression. This reconciles the accepted genetic and evolutionary basis of cancers with the observed polyclonality in tumours. Here, we provide a conceptual basis for examining the importance of cancer cell–cancer cell interactions to the behaviour of tumours and propose specific mechanisms by which clonal diversity in tumours, including that provided by EMTs, can drive the progression of tumours to malignancy.





Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial-mesenchymal transition
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194:23–28
Cahill DP, Kinzler KW, Vogelstein B et al (1999). Genetic instability and darwinian selection in tumours. Trends Cell Biol 9:M57–60
Hay ED (1995). An overview of epithelio-mesenchymal transformation. Acta Anat 154:8–20
Thompson EW, Newgreen DF, Tarin D (2005) Carcinoma invasion and metastasis:a role for epithelial–mesenchymal transition? Cancer Res 65:5991–5995; discussion 5995
Lebret SC, Newgreen DF, Thompson EW et al (2007) Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res 9:R19
Radisky DC, Levy DD, Littlepage LE et al (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123–127
Lebret SC, Newgreen DF, Waltham MC et al (2006) Myoepithelial molecular markers in human breast carcinoma PMC42-LA cells are induced by extracellular matrix and stromal cells. In Vitro Cell Dev Biol Anim 42:298–307
Chaffer CL, Brennan JP, Slavin JL et al (2006) Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis:role of fibroblast growth factor receptor-2. Cancer Res 66:11271–11278
Chaffer CL, Thompson EW, Williams ED (2007) Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 185:7–19
Choi KH, Chen CJ, Kriegler M et al (1988) An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell 53:519–529
Scotto KW (2003) Transcriptional regulation of ABC drug transporters. Oncogene 22:7496–7511
Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling:tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309
Sissung TM, Price DK, Sparreboom A et al (2006) Pharmacogenetics and regulation of human cytochrome P450 1B1:implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res 4:135–150
Chien WM, Rabin S, Macias E et al (2006) Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc Natl Acad Sci USA 103:4122–4127
Garcia SB, Novelli M, Wright NA (2000) The clonal origin and clonal evolution of epithelial tumours. Int J Exp Pathol 81:89–116
Going JJ (2003) Epithelial carcinogenesis: challenging monoclonality. J Pathol 200:1–3
Heim S, Teixeira MA, Pandis N (2001) Are some breast carcinomas polyclonal in origin?. J Pathol 194:395–397
Teixeira MR, Tsarouha H, Kraggerud SM et al (2001) Evaluation of breast cancer polyclonality by combined chromosome banding and comparative genomic hybridization analysis. Neoplasia 3:204–214
Winton DJ, Blount MA, Ponder BA (1989) Polyclonal origin of mouse skin papillomas. Br J Cancer 60:59–63
Cheng L, Gu J, Ulbright TM et al (2002) Precise microdissection of human bladder carcinomas reveals divergent tumor subclones in the same tumor. Cancer 94:104–110
Asplund A, Sivertsson A, Backvall H et al (2005) Genetic mosaicism in basal cell carcinoma. Exp Dermatol 14:593–600
Novelli MR, Williamson JA, Tomlinson IP et al (1996) Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 272:1187–1190
Konishi N, Hiasa Y, Matsuda H et al (1995) Intratumor cellular heterogeneity and alterations in ras oncogene and p53 tumor suppressor gene in human prostate carcinoma. Am J Pathol 147:1112–1122
Giaretti W, Monaco R, Pujic N et al (1996) Intratumor heterogeneity of K-ras2 mutations in colorectal adenocarcinomas: association with degree of DNA aneuploidy. Am J Pathol 149:237–245
Alvarado C, Beitel LK, Sircar K et al (2005) Somatic mosaicism and cancer: a micro-genetic examination into the role of the androgen receptor gene in prostate cancer. Cancer Res 65:8514–8518
Gonzalez-Garcia I, Sole RV, Costa J (2002) Metapopulation dynamics and spatial heterogeneity in cancer. Proc Natl Acad Sci USA 99:13085–13089
Agar NS, Halliday GM, Barnetson RS et al (2004) The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA 101:4954–4959
Jones AC, Sampson JR, Cheadle JP (2001) Low level mosaicism detectable by DHPLC but not by direct sequencing. Hum Mutat 17:233–234
Backvall H, Asplund A, Gustafsson A et al (2005) Genetic tumor archeology: microdissection and genetic heterogeneity in squamous and basal cell carcinoma. Mutat Res 571:65–79
Novelli M, Cossu A, Oukrif D et al (2003) X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc Natl Acad Sci USA 100:3311–3314
Going JJ, Abd El-Monem HM, Craft JA (2001) Clonal origins of human breast cancer. J Pathol 194:406–412
Lyons JG, Siew K, O’Grady RL (1989) Cellular interactions determining the production of collagenase by a rat mammary carcinoma cell line. Int J Cancer 43:119–125
Martorana AM, Zheng G, Crowe TC et al (1998) Epithelial cells upregulate matrix metalloproteinases in cells within the same mammary carcinoma that have undergone an epithelial-mesenchymal transition. Cancer Res 58:4970–4979
Zheng G, Lyons JG (2002) Cyclosporin A improves the selection of cells transfected with the puromycin acetyltransferase gene. BioTechniques 33:32–34
Israel L (1990) Accelerated genetic destabilization and dormancy: two distinct causes of resistance in metastatic cells; clinical magnitude, therapeutic approaches. Clin Exp Metastasis 8:1–11
Michor F, Iwasa Y, Nowak MA (2004) Dynamics of cancer progression. Nat Rev Cancer 4:197–205
Blanpain C, Horsley V, Fuchs E (2007) Epithelial stem cells: turning over new leaves. Cell 128:445–458
Willipinski-Stapelfeldt B, Riethdorf S, Assmann V et al (2005) Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 11:8006–8014
Van den Broecke C, Vleminckx K, De Bruyne G et al (1996) Morphotypic plasticity in vitro and in nude mice of epithelial mouse mammary cells (NMuMG) displaying an epithelioid (e) or a fibroblastic (f) morphotype in culture. Clin Exp Metastasis 14:282–296
Agiostratidou G, Hulit J, Phillips GR et al (2007) Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia 12:127–133
Petersen OW, Nielsen HL, Gudjonsson T et al (2003) Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol 162:391–402
Maniotis AJ, Folberg R, Hess A et al (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155:739–752
Tse JC, Kalluri R (2007) Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101:816–829
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39:305–318
Lee JM, Dedhar S, Kalluri R et al (2006) The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 172:973–981
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
Boyer B, Roche S, Denoyelle M et al (1997) Src and ras are involved in separate pathways in epithelial cell scattering. EMBO J 16:5904–5913
Oft M, Akhurst RJ, Balmain A (2002) Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol 4:487–494
Chua HL, Bhat-Nakshatri P, Clare SE et al (2007) NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26:711–724
Frank SA (2003) Somatic mosaicism and cancer: inference based on a conditional Luria-Delbruck distribution. J Theor Biol 223:405–412
Barrett MT, Sanchez CA, Prevo LJ et al (1999) Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Genet 22:106–109
Kurose K, Gilley K, Matsumoto S et al (2002) Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet 32:355–357
Fidler IJ (1973) Selection of successive tumour lines for metastasis. Nat New Biol 242:148–149
Poste G, Doll J, Fidler IJ (1981) Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc Natl Acad Sci USA 78:6226–6230
Talmadge JE, Fidler IJ (1982) Cancer metastasis is selective or random depending on the parent tumour population. Nature 297:593–594
Talmadge JE, Wolman SR, Fidler IJ (1982) Evidence for the clonal origin of spontaneous metastases. Science 217:361–363
Waghorne C, Thomas M, Lagarde A et al (1988) Genetic evidence for progressive selection and overgrowth of primary tumors by metastatic cell subpopulations. Cancer Res 48:6109–6114
Price JE, Bell C, Frost P (1990) The use of a genotypic marker to demonstrate clonal dominance during the growth and metastasis of a human breast carcinoma in nude mice. Int J Cancer 45:968–971
Theodorescu D, Cornil I, Sheehan C et al (1991) Dominance of metastatically competent cells in primary murine breast neoplasms is necessary for distant metastatic spread. Int J Cancer 47:118–123
Zhang X, Su L, Pirani AA et al (2006) Understanding metastatic SCCHN cells from unique genotypes to phenotypes with the aid of an animal model and DNA microarray analysis. Clin Exp Metastasis 23:209–222
Chambers AF, Wilson S (1988) Use of NeoR B16F1 murine melanoma cells to assess clonality of experimental metastases in the immune-deficient chick embryo. Clin Exp Metastasis 6:171–182
Weiss L, Holmes JC, Ward PM (1983) Do metastases arise from pre-existing subpopulations of cancer cells? Br J Cancer 47:81–89
Vaage J (1988) Metastasizing potentials of mouse mammary tumors and their metastases. Int J Cancer 41:855–858
Samiei M, Waghorne CG (1991) Clonal selection within metastatic SP1 mouse mammary tumors is independent of metastatic potential. Int J Cancer 47:771–775
Moffett BF, Baban D, Bao L et al (1992) Fate of clonal lineages during neoplasia and metastasis studied with an incorporated genetic marker. Cancer Res 52:1737–1743
Poste G, Tzeng J, Doll J et al (1982) Evolution of tumor cell heterogeneity during progressive growth of individual lung metastases. Proc Natl Acad Sci USA 79:6574–6578
Hill RP, Chambers AF, Ling V et al (1984) Dynamic heterogeneity:rapid generation of metastatic variants in mouse B16 melanoma cells. Science 224:998–1001
Nanni P, De Giovanni C, Lollini PL et al (1986) Clones with different metastatic capacity and variant selection during metastasis:a problematic relationship. J Natl Cancer Inst 76:87–93
Miller BE, Miller FR, Wilburn D et al (1988) Dominance of a tumor subpopulation line in mixed heterogeneous mouse mammary tumors. Cancer Res 48:5747–5753
Miller BE, McInerney D, Jackson D et al (1986) Metabolic cooperation between mouse mammary tumor subpopulations in three-dimensional collagen gel cultures. Cancer Res 46:89–93
Miller FR, McEachern D, Miller BE (1990) Efficiency of communication between tumour cells in collagen gel cultures. Br J Cancer 62:360–363
Miller F (1983) Tumor subpopulation interactions in metastasis. Invasion Metastasis 3:234–242
Montaner S, Sodhi A, Molinolo A et al (2003) Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23–36
Maley CC, Galipeau PC, Finley JC et al (2006) Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet 38:468–473
Alarcon T, Byrne HM, Maini PK (2005) A multiple scale model for tumor growth. Multiscale Model Sim 3:440–475
Cristini V, Frieboes HB, Gatenby R et al (2005) Morphologic instability and cancer invasion. Clin Cancer Res 11:6772–6779
Anderson AR (2005) A hybrid mathematical model of solid tumour invasion:the importance of cell adhesion. Math Med Biol 22:163–186
Anderson AR, Weaver AM, Cummings PT et al (2006) Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127:905–915
Steinberg MS (1962) On the mechanism of tissue reconstruction by dissociated cells, III. Free energy relations and the reorganization of fused, heteronomic tissue fragments. Proc Natl Acad Sci USA 48:1769–1776
Steinberg MS (1962) On the mechanism of tissue reconstruction by dissociated cells. I. Population kinetics, differential adhesiveness. and the absence of directed migration. Proc Natl Acad Sci USA 48:1577–1582
Armstrong NJ, Painter KJ, Sherratt JA (2006) A continuum approach to modelling cell-cell adhesion. J Theor Biol 243:98–113
Coskun H, Li Y, Mackey MA (2007) Ameboid cell motility:a model and inverse problem, with an application to live cell imaging data. J Theor Biol 244:169–179
Gracheva ME, Othmer HG (2004) A continuum model of motility in ameboid cells. Bull Math Biol 66:167–193
Acknowledgments
This work was supported by grants from the Sydney Cancer Centre Foundation and Cancer Council NSW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyons, J.G., Lobo, E., Martorana, A.M. et al. Clonal diversity in carcinomas: its implications for tumour progression and the contribution made to it by epithelial-mesenchymal transitions. Clin Exp Metastasis 25, 665–677 (2008). https://doi.org/10.1007/s10585-007-9134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-007-9134-2