Abstract
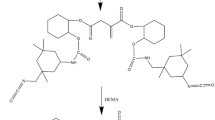
Nano-sized silica particles were used as fillers for acrylates. For favourable embedding of the silica particles within the acrylate matrix the surface of the fillers was chemically modified by the reaction with methacryloxypropyl trimethoxysilane. A polysiloxane shell is formed as product of an acid catalysed condensation of the organosilanes. This shell is linked to the silica via reaction with the surface silanol groups of the silica. The appearance of covalent Si−O−Si−R bonds formed either by the condensation of silanes and/or by the reaction with Si−OH groups on the silica was demonstrated by infrared, multinuclear MAS NMR and mass spectroscopy.
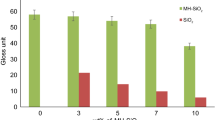
A typically acrylate-nanocomposite formulation contains up to 35 wt. % of polysiloxane covered nano-sized silica. The polysiloxane shell still carries methacrylate groups, which can be copolymerised with acrylates. After UV or electron beam curing of such formulations polyacrylate nanocomposites are obtained. These composite materials exhibit markedly improved properties as compared to pure polymers, e.g. an increased modulus and heat resistance, improved scratch and abrasion resistance as well as reduced gas permeabilities. This makes them very promising as coatings for technical applications.
Similar content being viewed by others
References
Technical bulletin AEROSIL # 11 from Degussa
Brand M., et al.: Z. Naturforsch. 54b (1999) 155.
Beari F. et al.: J. Organomet. Chem. 625 (2001) 208.
Vidal A. and J. B. Donnet: Bull. Soc. Chim. Fr. 1985, No. 6, 1088.
Hommel H., Touhami A. and A. P. Legrand: Makromol. Chem. 194 (1993) 879.
Albert K., Brindle R., Schmid J., Buszewski R., and Bayer E.: Chromatographia 38 (1994) 283.
Mathew J. P. and Srinivasan M.: Polym. Int. 29 (1992) 179.
Bauer F. et al.: Macromol. Chem. 201 (2000) 2654.
Gläsel H.-J. et al.: Macromol. Chem. 201 (2000) 2765.
Pfeifer H. and Winkler H.: Magnetic Resonance Methods, in: Preparative Chemistry Using Supported Reagents, Academic Press, New York, 1987, p. 151.
Bayer E., Albert K., Reiners J., Nieder M. and Müller D.: J. Chrom. 264 (1983) 197.
Sindorf D. W. and Maciel G. E.: J. Amer. Chem. Soc. 105 (1983) 3767.
Albert K., Bayer E. and Pfleiderer B.: J. Chrom. 506 (1990) 343.
Lewis L. N. and Katsamberis D.: J. Appl. Poly. Sci. 42 (1991) 155.
Mathias J. and Wannemacher G.: J. Coll. Interf. Sci. 125 (1988) 61.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tauber, A., Hartmann, E., Gläsel, H.J. et al. UV and electron beam crosslinked polyacrylate nanocomposites and their applications. Czech J Phys 53 (Suppl 1), A355–A367 (2003). https://doi.org/10.1007/s10582-003-0045-4
Issue Date:
DOI: https://doi.org/10.1007/s10582-003-0045-4