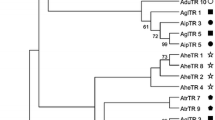
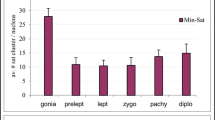
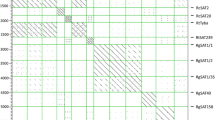
Abstract
African great apes have large constitutive heterochromatin (C-band) blocks in subtelomeric regions of the majority of their chromosomes, but humans lack these. Additionally, the chimpanzee meiotic cell division process demonstrates unique partial terminal associations in the first meiotic prophase (pachytene). These are likely formed as a result of interaction among subtelomeric C-band blocks. We thus conducted an extensive study to define the features in the subtelomeric heterochromatic regions of chimpanzee chromosomes undergoing mitotic metaphase and meiotic cell division. Molecular cytogenetic analyses with probes of both subterminal satellite DNA (a main component of C-band) and rDNA demonstrated principles of interaction among DNA arrays. The results suggest that homologous and ectopic recombination through persistent subtelomeric associations (post-bouquet association observed in 32% of spermatocytes in the pachytene stage) appears to create variability in heterochromatin patterns and simultaneously restrain subtelomeric genome polymorphisms. That is, the meeting of non-homologous chromosome termini sets the stage for ectopic pairing which, in turn, is the mechanism for generating variability and genomic dispersion of subtelomeric C-band blocks through a system of concerted evolution. Comparison between the present study and previous reports indicated that the chromosomal distribution rate of sutelomeric regions seems to have antagonistic correlation with arm numbers holding subterminal satellite blocks in humans, chimpanzees, and gorillas. That is, the increase of subterminal satellite blocks probably reduces genomic diversity in the subtelomeric regions. The acquisition vs. loss of the subtelomeric C-band blocks is postulated as the underlying engine of this chromosomal differentiation yielded by meiotic chromosomal interaction.






Similar content being viewed by others
Abbreviations
- C-band:
-
Constitutive heterochromatin
- CeC:
-
Centromeric C-band
- DAPI:
-
4′-6-Diamidino-2-phenylindole
- FISH:
-
Fluorescent in situ hybridization
- NaC:
-
NOR-adjoining C-band
- NOR:
-
Nucleolus organizer region
- SCB:
-
Subterminal constitutive heterochromatin block
- StC:
-
Subterminal C-band
- StSat:
-
Subterminal satellite
References
Alkan C, Ventura M, Achiaiacono N, Rocchi M, Sahinalp SC, Eichler EE (2007) Organization and evolution of primate centromeric DNA from whole-genome shotgun sequence data. PLoS Comput Biol 3(9):e181
Allshire RC, Madhani HD (2018) Ten principles of heterochromatin formation and function. Nature reviews. Mol Cell Biol 19:229–244
Arnheim N, Krystal M, Schmickel R, Wilson G, Ryder O, Zimmer E (1980) Molecular evidence for genetic exchanges among ribosomal genes on nonhomologous chromosomes in man and apes. Proc Natl Acad Sci U S A 77:7323–7327
Assum G, Gartmann C, Schempp W, Wohr G (1994) Evolution of the chAB4 multisequence family in primates. Genomics 21:34–41
Cheng Z, Ventura M, She X, Khaitovich P, Graves T, Osoegawa K, Church D, DeJongP WRK, Paab S et al (2005) A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature 437:88–93
Dover G, Coen E (1981) Springcleaning ribosomal DNA: a model for multigene evolution. Nature 290:731–732
Drets M, Stool M (1974) C-banding and non-homologous associations in Gryllus argentinus. Chromosoma 48:367–390
Driscoll DJ, Palmer CG, Melman A (1979) Nonhomologous associations of C-heterochromatin at human male meiotic prophase. Cytogenet Cell Genet 23:23–32
Guillén AKZ, Hirai Y, Tanoue T, Hirai H (2004) Transcriptional repression mechanisms of nucleolus organizer regions (NORs) in humans and chimpanzees. Chromosom Res 12:225–237
Haaf T, Schmid M (1987) Chromosome heteromorphisms in the gorilla karyotype. J Hered 78:287–292
Harding RM, Boyce AJ, Clegg JB (1992) The evolution of tandemly repetitive DNA: recombination riles. Genetics 132:847–859
Hirai H (2001) Relationship of telomere sequence and constitutive heterochromatin in the human and apes as detected by PRINS. Methods Cell Sci 23:29–35
Hirai H, Hirai Y (2004) FISH mapping for helminth genome. In: Melville SE (ed) Methods in molecular biology. Humana Press, Totowa, pp 379–394
Hirai H, Hirata M, Aoki Y, Tanaka M, Imai HT (1996) Chiasma analyses of the parasite flukes, Schistosoma and Paragonimus (Trematoda), by using the chiasma distribution graph. Gene Genet Syst 71:181–188
Hirai H, Taguchi T, Godwin K (1999) Genomic differentiation of 18S ribosomal DNA and β-satellite DNA in the hominoid and its evolutionary aspects. Chromosom Res 7:531–540
Hirai H, Matsubayashi K, Kumazaki K, Kato A, Maeda N, Kim H-S (2005) Chimpanzee chromosomes: retrotransposable compound repeat DNA organization (RCRO) and its influence on meiotic prophase and crossing-over. Cytogenet Genome Res 108:248–254
Imai HT (2018) On-line monograph: the minimum interaction theory. https://minimum-interaction-theory.jimdofree.com/. Accessed 15 Feb 2018
Imai HT, Taylor RW, Crosland MW, Crozier RH (1988) Modes of spontaneous chromosomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn J Genet 63:159–185
Janssen A, Colmenares SU, Karpen GH (2018) Heterochromatin: guardian of the genome. Annu Rev Cell Dev Biol 34:265–288
John B (1988) The biology of heterochromatin. In: Verma I, Ram S (eds) Heterochromatin. Cambridge University Press, New York, pp 1–147
John B, King M (1980) Heterochromatin variation in Cryptobothrus chrysophorus III. Synthetic hybrids. Chromosoma 78:165–186
Kasai F, Takahashi E, Koyama K, Terao K, Suto Y, Tokunaga K, Nakamura Y, Hirai M (2000) Comparative FISH mapping of the ancestral fusion point of human chromosome 2. Chromosom Res 8:727–7235
Koga A, Notohara M, Hirai H (2011) Evolution of subterminal satellite (StSat) repeats in hominids. Genetica 139:167–175
Linardopoulou EV, Williams EM, Fan Y, Freiedman C, Young JM, Trask BJ (2005) Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 437:94–100
Locke DP, Hillier LW, Warren WC et al (2011) Comparative and demographic analysis of orang-utan genomes. Nature 469:529–533
Luke S, Verma RS (1992) Origin of human chromosome 2. Nat Genet 2:11–12
Luke S, Verma RS (1995) Human (Homo sapiens) and chimpanzee (Pan troglodytes) share similar ancestral centromeric alpha satellite DNA sequences but other fractions of heterochromatin differ considerably. Am J Phys Anthropol 96:63–71
Maeda N, Smithies O (1986) The evolution of multigene families: human haptoglobin genes. Ann Rev Genet 20:81–108
Marks J (1985) C-band variability in the common chimpanzee, Pan troglodytes. J Hum Evol 14:669–675
Marks J (1993) Hominoid heterochromatin: terminal C-bands as a complex genetic trait linking chimpanzee and gorilla. Am J Phys Anthropol 90:237–246
Ohta T (1987) Simulating evolution by gene duplication. Genetics 115:207–213
Primate Research Institute, Kyoto University (2002) Guide for the care and use of laboratory primates, 2nd edn. Kyoto University, Inuyam
Royle NJ, Baird DM, Jeffreys AJ (1994) A subterminal satellite located adjacent to telomeres in chimpanzees is absent from the human genome. Nat Genet 6:52–56
Schempp W, Zeitler S, Reitschel W (1998) Chromosomal localization of rDNA in the gorilla. Cytogenet Cell Genet 80:185–187
Schlötterrere C, Tautz D (1994) Chromosomal homogeneity of Drosophila ribosomal DAN arrays suggested intrachromosomal exchanges drive concerted evolution. Curr Biol 4:777–783
Schmid M, Krone W, Vogel W (1974) On the relationship between the frequency of association and the nuclear constriction of individual acrocentric chromosomes. Humangenetik 23:267–277
Schmid M, Grunert D, Haaf T, Engel W (1983a) A direct demonstration of somatically paired heterochromatin of human chromosomes. Cytogenet Cell Genet 36:554–561
Schmid M, Müller HJ, Stasch S, Engel W (1983b) Silver staining of nucleolus organizer regions during human spermatogenesis. Human Genet 64:363–370
Schweizer D, Loidl J (1987) A model for heterochromatin dispersion and the evolution of C-band patterns. Chromosomes Today 9:61–74
Stanyon R, Chiarelli B, Gottlieb K, Patton W (1986) The phylogenetic and taxonomic status of Pan paniscus: a chromosomal perspective. Am J Phys Anthropol 69:489–498
Styack SM, Shearer LA, Lohmiller L, Anderson LK (2017) Meiotic crossing over in maize knob heterochromatin. Genetics 205:1101–1112
Sumner AT (1990) Chromosome banding. Unwin Hyman, London
Tuck-Muller CM, Bordson BL, Varela M, Bennet JW (1984) NOR association with heterochromatin. Cytogenet Cell Genet 38:165–170
Ventura M, Catacchio CR, Alkan C, Marques-Bonet T, Sajjadian S, Graves TA, Hormozdiari F, Navarro A, Malig M, Baker C, Lee C, Turner EH, Chen L, Kidd JM, Archidiacono N, Shendure J, Wilson RK, Eichler EE (2011) Gorilla genome structural variation reveals evolutionary parallelisms with chimpanzee. Genome Res 21:1640–1649
Yunis JJ, Prakash O (1982) The origin of man: a chromosomal pictorial legacy. Science 215:1525–1530
Zang KD, Back E (1968) Quantitative studies on the arrangement of human metaphase chromosomes. I. Individual features in the association pattern of the acrocentric chromosomes of normal males and females. Cytogenetics 7:455–470
Zimmer EA, Martin SL, Beverley SM, Kan YW, Wilson AC (1980) Rapid duplication and loss of genes coding for the α chains of hemoglobin. Proc Natl Acad Sci U S A 77:2158–2162
Acknowledgments
We thank the staff members of the Center of Human Evolution Modeling Research, Primate Research Institute, and the Kumamoto Sanctuary, Wildlife Research Center, Kyoto University, for their technical assistance. We are grateful to the Japan Monkey Center and Ishikawa Zoo for supplying blood samples of bonobo and gorilla, respectively.
Funding
This research was supported by grants from the Japan Society for the Promotion of Science (JSPS; 15H04427 to AK, 20405016 and 22247037 to HH), the Global Center of Excellence of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT; Program A06, Kyoto University), the Unit of Human-Nature Interlaced Life Science, Kyoto University Research Coordination Alliance, and the Kyoto University Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal ethics approval was obtained from the Monkey Care and Use Committee of Primate Research Institute, Kyoto University (approvals 07–1632, 08–1703, 09–1787, 2010–084).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Tatsuo Fukagawa
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Hirai, H., Hirai, Y., Udono, T. et al. Structural variations of subterminal satellite blocks and their source mechanisms as inferred from the meiotic configurations of chimpanzee chromosome termini. Chromosome Res 27, 321–332 (2019). https://doi.org/10.1007/s10577-019-09615-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-019-09615-z