Abstract
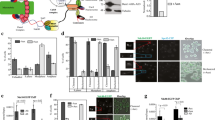
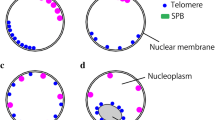
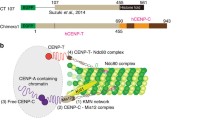
In yeast and animals, Nuclear Division Cycle 80 (NDC80) is an important kinetochore protein that binds to microtubules and mediates chromosome movement. Its localization pattern is unusual, since it is generally not viewed as either an inner (centromeric chromatin) or outer (regulatory) component of the kinetochore. Here we report the characterization of NDC80 in a higher plant. By taking advantage of the large meiotic kinetochores of maize, we were able to show that NDC80 localizes outside of the constitutive kinetochore protein CENP-C. Further, a detailed analysis of mitosis indicates that NDC80 is stably present on kinetochores throughout the cell cycle. The quantity of NDC80 positively correlates with measured quantities of DNA and CENP-C, suggesting that NDC80 rapidly associates with DNA following replication and is stably maintained at centromeres during cell division. The data suggest that in plants NDC80 is on par with ‘foundation’ kinetochore proteins such as CENH3 and CENP-C.
Similar content being viewed by others
References
Amor DJ, Kalitsis P, Sumer H, Choo KH (2004) Building the centromere: from foundation proteins to 3D organization. Trends Cell Biol 14: 359–368.
Asai DJ, Brokaw CJ, Thompson WC, Wilson L (1982) Two different monoclonal antibodies to tubulin inhibit the bending of reactivated sea urchin spermatozoa. Cell Motil 2: 599–614.
Asakawa H, Hayashi A, Haraguchi T, Hiraoka Y (2005) Dissociation of the Nuf2-Ndc80 complex releases centromeres from the spindle-pole body during meiotic prophase in fission yeast. Mol Biol Cell 16: 2325–2338.
Bharadwaj R, Qi W, Yu H (2004) Identification of two novel components of the human NDC80 kinetochore complex. J Biol Chem 279: 13076–13085.
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127: 983–997.
Chen Y, Riley DJ, Chen PL, Lee WH (1997) HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol Cell Biol 17: 6049–6056.
Dawe RK, Sedat JW, Agard DA, Cande WZ (1994) Meiotic chromosome pairing in maize is associated with a novel chromatin organization. Cell 76: 901–912.
Dawe RK, Reed LM, Yu HG, Muszynski MG, Hiatt EN (1999) A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore. Plant Cell 11: 1227–1238.
DeLuca JG, Dong Y, Hergert P et al. (2005) Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell 16: 519–531.
DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED (2006) Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127: 969–982.
Desai A, Rybina S, Muller-Reichert T et al. (2003) KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev 17: 2421–2435.
De Wulf P, McAinsh AD, Sorger PK (2003) Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev 17: 2902–2921.
Fukagawa T (2004) Assembly of kinetochores in vertebrate cells. Exp Cell Res 296: 21–27.
Granger C, Cyr R (2001) Use of abnormal preprophase bands to decipher division plane determination. J Cell Sci 114: 599–607.
He X, Rines DR, Espelin CW, Sorger PK (2001) Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell 106: 195–206.
Hiatt EN, Kentner EK, Dawe RK (2002) Independently regulated neocentromere activity of two classes of tandem repeat arrays. Plant Cell 14: 407–420.
Hori T, Haraguchi T, Hiraoka Y, Kimura H, Fukagawa T (2003) Dynamic behavior of Nuf2-Hec1 complex that localizes to the centrosome and centromere and is essential for mitotic progression in vertebrate cells. J Cell Sci 116: 3347–3362.
Houben A, Schubert I (2003) DNA and proteins of plant centromeres. Curr Opin Plant Biol 6: 554–560.
Janke C, Ortiz J, Lechner J et al. (2001) The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J 20: 777–791.
Kline-Smith SL, Sandall S, Desai A (2005) Kinetochore–spindle microtubule interactions during mitosis. Curr Opin Cell Biol 17: 35–46.
Lai J, Dey N, Kim CS et al. (2004) Characterization of the maize endosperm transcriptome and its comparison to the rice genome. Genome Res 14: 1932–1937.
Lermontova I, Schubert V, Fuchs J, Klatte S, Macas J, Schubert I (2006) Loading of Arabidopsis centromeric histone CENH3 occurs mainly during G2 and requires the presence of the histone fold domain. Plant Cell 18: 2443–2451.
Mao Y, Desai A, Cleveland DW (2005) Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J Cell Biol 170: 873–880.
McAinsh AD, Tytell JD, Sorger PK (2003) Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol 19: 519–539.
McCleland ML, Gardner RD, Kallio MJ et al. (2003) The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev 17: 101–114.
Meraldi P, McAinsh AD, Rheinbay E, Sorger PK (2006) Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol 7: R23.
Mikami Y, Hori T, Kimura H, Fukagawa T (2005) The functional region of CENP-H interacts with the Nuf2 complex that localizes to centromere during mitosis. Mol Cell Biol 25: 1958–1970.
Pidoux AL, Allshire RC (2004) Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res 12: 521–534.
Sato H, Shibata F, Murata M (2005) Characterization of a Mis12 homologue in Arabidopsis thaliana. Chromosome Res 13: 827–834.
Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S (2002) Centromeric localization and adaptive evolution of an Arabidopsis histone H3 variant. Plant Cell 14: 1053–1066.
Talbert PB, Bryson TD, Henikoff S (2004) Adaptive evolution of centromere proteins in plants and animals. J Biol 3: 18.
Wei RR, Al-Bassam J, Harrison SC (2007) The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol 14: 54–59.
Westermann S, Cheeseman IM, Anderson S, Yates JR III, Drubin DG, Barnes G (2003) Architecture of the budding yeast kinetochore reveals a conserved molecular core. J Cell Biol 163: 215–222.
Wigge PA, Kilmartin JV (2001) The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol 152: 349–360.
Yu HG, Muszynski MG, Dawe RK. (1999) The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J Cell Biol 145: 425–435.
Yu HG, Hiatt EN, Dawe RK (2000) The plant kinetochore. Trends Plant Sci 5: 543–547.
Zhang X, Li X, Marshall JB, Zhong CX, Dawe RK (2005) Phosphoserines on maize CENTROMERIC HISTONE H3 and histone H3 demarcate the centromere and pericentromere during chromosome segregation. Plant Cell 17: 572–583.
Zheng L, Chen Y, Lee WH (1999) Hec1p, an evolutionarily conserved coiled-coil protein, modulates chromosome segregation through interaction with SMC proteins. Mol Cell Biol 19: 5417–5428.
Zhong CX, Marshall JB, Topp C et al. (2002) Centromeric retroelements and satellites interact with maize kinetochore protein CENH3. Plant Cell 14: 2825–2836.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, Y., Dawe, R.K. Maize NDC80 is a constitutive feature of the central kinetochore. Chromosome Res 15, 767–775 (2007). https://doi.org/10.1007/s10577-007-1160-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-007-1160-z